极光激酶 A 介导的磷酸化引发 Rab1A 结构改变,从而增强有丝分裂过程中 ER 的复杂性
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
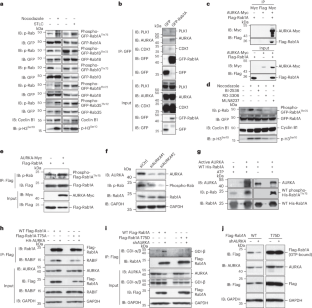
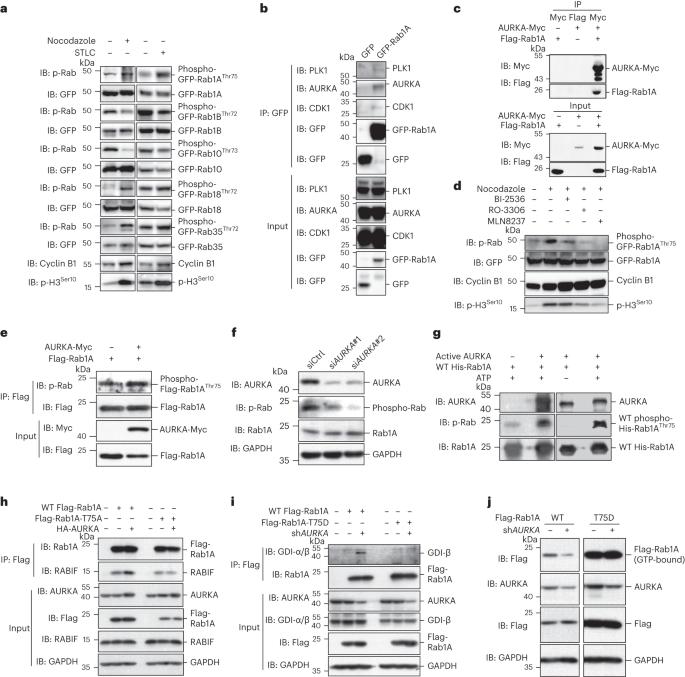
内质网(ER)的形态重排对于后生动物的有丝分裂至关重要。然而,有丝分裂信号如何重塑内质网仍不清楚。在这里,我们报告了有丝分裂极光激酶 A(AURKA)利用小 GTP 酶 Rab1A 来指导 ER 重塑。在有丝分裂过程中,AURKA 在 Thr75 处磷酸化 Rab1A。结构分析表明,Thr75 磷酸化阻止了 Rab1A 与 GDP 解离抑制剂(GDI)的相互作用,从而使 Rab1A 处于持续活跃状态。活化的 Rab1A 保留在 ER 上,并诱导 ER 塑形蛋白 RTN 和 REEPs 的寡聚化,最终引发 ER 复杂性的增加。在从秀丽隐杆线虫、果蝇到哺乳动物的各种模型中,通过基因修饰抑制 Rab1AThr75 磷酸化会破坏 ER 重塑。因此,我们的研究揭示了解释有丝分裂激酶如何控制ER重塑的进化保守机制,并发现了Rab GTP酶在分裂期的关键功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Aurora kinase A-mediated phosphorylation triggers structural alteration of Rab1A to enhance ER complexity during mitosis
Morphological rearrangement of the endoplasmic reticulum (ER) is critical for metazoan mitosis. Yet, how the ER is remodeled by the mitotic signaling remains unclear. Here, we report that mitotic Aurora kinase A (AURKA) employs a small GTPase, Rab1A, to direct ER remodeling. During mitosis, AURKA phosphorylates Rab1A at Thr75. Structural analysis demonstrates that Thr75 phosphorylation renders Rab1A in a constantly active state by preventing interaction with GDP-dissociation inhibitor (GDI). Activated Rab1A is retained on the ER and induces the oligomerization of ER-shaping protein RTNs and REEPs, eventually triggering an increase of ER complexity. In various models, from Caenorhabditis elegans and Drosophila to mammals, inhibition of Rab1AThr75 phosphorylation by genetic modifications disrupts ER remodeling. Thus, our study reveals an evolutionarily conserved mechanism explaining how mitotic kinase controls ER remodeling and uncovers a critical function of Rab GTPases in metaphase. Here, the authors show how Aurora kinase A (AURKA) employs Rab1a to direct ER remodeling. Activated Rab1A is retained on the ER and directly interacts with the RTN/REEP ER-shaping machinery to promote its oligomerization, eventually triggering an increase of ER complexity during mitosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


