Stephen Okiemute Akpasi, Yusuf Makarfi Isa, Thembisile Patience Mahlangu, Sammy Lewis Kiambi
下载PDF
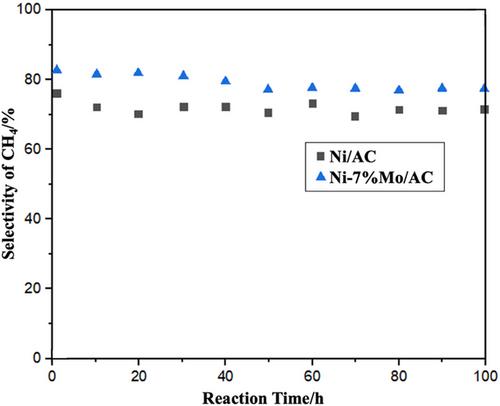
{"title":"活性炭支撑镍基催化剂添加钼对二氧化碳甲烷化的影响","authors":"Stephen Okiemute Akpasi, Yusuf Makarfi Isa, Thembisile Patience Mahlangu, Sammy Lewis Kiambi","doi":"10.1002/ghg.2257","DOIUrl":null,"url":null,"abstract":"<p>Recently, CO<sub>2</sub> methanation has become a technique that aims to reduce anthropogenic CO<sub>2</sub> emissions by converting CO<sub>2</sub> captured from stationary and mobile sources and H<sub>2</sub> produced from renewable sources into CH<sub>4</sub>. Due to their excellent performance-to-cost ratio, Ni-based catalysts were frequently used in such conversions. The main drawbacks, however, are that Ni has the propensity to aggregate and deposit carbon during the high-temperature reaction. These issues can be partially resolved by including a support (e.g., MOF, zeolite, activated carbon, etc.) and a second transition metal (e.g., Mo, Co, or Fe) in Ni-based catalysts. Therefore, the activity of Ni-based catalysts at low temperatures needs to be improved. In this study, a series of mesoporous activated carbon (AC) supported bimetallic Ni–Mo catalysts (Ni–<i>x</i>Mo/AC, Ni = 13 wt.%, <i>x</i> = 5, 7, 9, 11 wt.%) were synthesized using the incipient wetness impregnation method. The effect of Mo content on the catalyst's activity was examined in a fixed-bed reactor. At 250–650°C, 1-atmosphere pressure, gas hourly space velocity (GHSV): 1200 mL h<sup>−1</sup> g<sup>−1</sup>, and H<sub>2</sub>/CO<sub>2</sub> ratio: 4:1, the catalytic efficiency of these catalysts was examined. The catalysts were analyzed using transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), X-ray powder diffraction (XRD), N<sub>2</sub>-physisorption, and scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM-EDX). Ni–7%Mo/AC catalyst showed the lowest carbon deposition rate, superior stability, and the best activity. The addition of Mo can improve the heat resistance of the Ni/AC catalyst and the interaction between the metal nickel and the support, which prevents the sintering of the catalyst. © 2023 Society of Chemical Industry and John Wiley & Sons, Ltd.</p>","PeriodicalId":12796,"journal":{"name":"Greenhouse Gases: Science and Technology","volume":"14 1","pages":"152-167"},"PeriodicalIF":2.8000,"publicationDate":"2023-12-26","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2257","citationCount":"0","resultStr":"{\"title\":\"Effects of molybdenum addition to activated carbon supported Ni-based catalysts for CO2 methanation\",\"authors\":\"Stephen Okiemute Akpasi, Yusuf Makarfi Isa, Thembisile Patience Mahlangu, Sammy Lewis Kiambi\",\"doi\":\"10.1002/ghg.2257\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Recently, CO<sub>2</sub> methanation has become a technique that aims to reduce anthropogenic CO<sub>2</sub> emissions by converting CO<sub>2</sub> captured from stationary and mobile sources and H<sub>2</sub> produced from renewable sources into CH<sub>4</sub>. Due to their excellent performance-to-cost ratio, Ni-based catalysts were frequently used in such conversions. The main drawbacks, however, are that Ni has the propensity to aggregate and deposit carbon during the high-temperature reaction. These issues can be partially resolved by including a support (e.g., MOF, zeolite, activated carbon, etc.) and a second transition metal (e.g., Mo, Co, or Fe) in Ni-based catalysts. Therefore, the activity of Ni-based catalysts at low temperatures needs to be improved. In this study, a series of mesoporous activated carbon (AC) supported bimetallic Ni–Mo catalysts (Ni–<i>x</i>Mo/AC, Ni = 13 wt.%, <i>x</i> = 5, 7, 9, 11 wt.%) were synthesized using the incipient wetness impregnation method. The effect of Mo content on the catalyst's activity was examined in a fixed-bed reactor. At 250–650°C, 1-atmosphere pressure, gas hourly space velocity (GHSV): 1200 mL h<sup>−1</sup> g<sup>−1</sup>, and H<sub>2</sub>/CO<sub>2</sub> ratio: 4:1, the catalytic efficiency of these catalysts was examined. The catalysts were analyzed using transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), X-ray powder diffraction (XRD), N<sub>2</sub>-physisorption, and scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM-EDX). Ni–7%Mo/AC catalyst showed the lowest carbon deposition rate, superior stability, and the best activity. The addition of Mo can improve the heat resistance of the Ni/AC catalyst and the interaction between the metal nickel and the support, which prevents the sintering of the catalyst. © 2023 Society of Chemical Industry and John Wiley & Sons, Ltd.</p>\",\"PeriodicalId\":12796,\"journal\":{\"name\":\"Greenhouse Gases: Science and Technology\",\"volume\":\"14 1\",\"pages\":\"152-167\"},\"PeriodicalIF\":2.8000,\"publicationDate\":\"2023-12-26\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2257\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Greenhouse Gases: Science and Technology\",\"FirstCategoryId\":\"93\",\"ListUrlMain\":\"https://scijournals.onlinelibrary.wiley.com/doi/10.1002/ghg.2257\",\"RegionNum\":4,\"RegionCategory\":\"环境科学与生态学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q3\",\"JCRName\":\"ENERGY & FUELS\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Greenhouse Gases: Science and Technology","FirstCategoryId":"93","ListUrlMain":"https://scijournals.onlinelibrary.wiley.com/doi/10.1002/ghg.2257","RegionNum":4,"RegionCategory":"环境科学与生态学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"ENERGY & FUELS","Score":null,"Total":0}
引用次数: 0
引用
批量引用


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


