探索富锂层状硫化物中阴离子氧化还原的瓶颈
IF 60.1
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 93
摘要
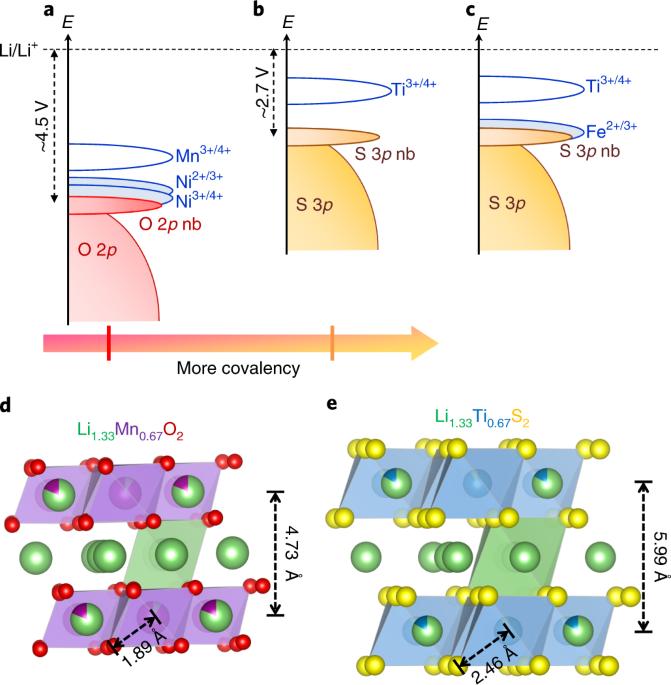
阴离子氧化还原化学已成为设计高能锂离子电池正极材料(如富锂层状氧化物)的新范例。然而,这些材料存在电压衰减、大滞后和动力学迟缓等问题,而这些问题都源于阴离子氧化还原活性本身。为了从根本上了解这些问题,我们决定通过设计新型富锂层状硫化物 Li1.33 - 2y/3Ti0.67 - y/3FeyS2 来解决配体问题,其中 y = 0.3 的成员由于阳离子(Fe2+/3+)和阴离子(S2-/Sn-,n <2)氧化还原过程的累积,显示出 ~245 mAh g-1 的持续可逆容量。此外,与富锂氧化物类似物相比,它的初始循环不可逆性可忽略不计,长时间循环后电压衰减减轻,电压滞后低,动力学速度快。因此,从氧配体转移到硫配体虽然会降低氧化还原电势和能量密度,但部分缓解了影响阴离子氧化还原的实际瓶颈。总之,这些硫化物为改善阴离子氧化还原电极的整体性能提供了化学线索,可指导我们最终利用氧氧化还原的能量优势。利用氧氧化还原是设计高能电池阴极材料的一种很有前景的方法。在此,Tarascon 及其同事报告了一类富锂层状硫化物,并揭示了硫氧化还原的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the bottlenecks of anionic redox in Li-rich layered sulfides
Anionic redox chemistry has emerged as a new paradigm to design higher-energy lithium ion-battery cathode materials such as Li-rich layered oxides. However, they suffer from voltage fade, large hysteresis and sluggish kinetics, which originate intriguingly from the anionic redox activity itself. To fundamentally understand these issues, we decided to act on the ligand by designing new Li-rich layered sulfides Li1.33 – 2y/3Ti0.67 – y/3FeyS2, among which the y = 0.3 member shows sustained reversible capacities of ~245 mAh g−1 due to cumulated cationic (Fe2+/3+) and anionic (S2−/Sn−, n < 2) redox processes. Moreover, its negligible initial cycle irreversibility, mitigated voltage fade upon long cycling, low voltage hysteresis and fast kinetics compare positively with its Li-rich oxide analogues. Moving from the oxygen ligand to the sulfur ligand thus partially alleviates the practical bottlenecks affecting anionic redox, although it penalizes the redox potential and energy density. Overall, these sulfides provide chemical clues to improve the holistic performance of anionic redox electrodes, which may guide us to ultimately exploit the energy benefits of oxygen redox. The utilization of oxygen redox is a promising way of designing high-energy cathode materials for batteries. Here, Tarascon and colleagues report a class of Li-rich layered sulfides and unravel the potential of sulfur redox.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


