具有羰基、酯和叠氮官能团的 1,2,3-三唑基取代 1,3,5- 三嗪的合成、表征和热分解性能
IF 3.9
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基于 2,4,6-三氮杂-1,3,5-三嗪与乙酰丙酮乙酰乙酸酯的烯胺叠氮加成有机催化反应,我们合成了一系列之前未知的单-、二-和三(1,2,3-三唑基)-取代的-1,3,5-三嗪,这些化合物额外带有羰基、酯基和叠氮基团。核磁共振(1H、13C)和红外光谱证明了所获化合物的结构,元素分析确认了其成分。借助差示扫描量热法(DSC)和热重分析法(TGA)耦合质谱法(TG-MS),我们获得了这些化合物的热行为和分解机理数据。我们发现,与三(1,2,3-三唑基)取代的 1,3,5 三嗪(分别为 180 ℃ 和 160 ℃)相比,二(1,2,3-三唑基)取代的 1,3,5 三嗪具有更高的热稳定性和更高的分解起始温度(220-250 ℃)。本文章由计算机程序翻译,如有差异,请以英文原文为准。

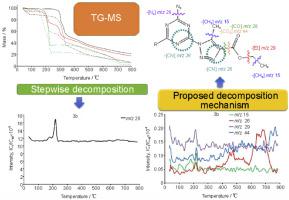
Synthesis, characterization, and thermal decomposition performance of 1,2,3-triazolyl-substituted 1,3,5-triazines with carbonyl, ester, and azide functional groups
Based on the organocatalytic reaction of enamine azide addition of 2,4,6-triazido-1,3,5-triazine to acetylacetone acetoacetic ester, we synthesized a series of previously unknown mono-, di-, and tri(1,2,3-triazolyl)-substituted-1,3,5-triazines that additionally carried carbonyl, ester, and azide groups. The structure of the obtained compounds was proved by NMR (1H, 13C) and IR spectroscopy, and the composition was confirmed by elemental analysis. With the aid of differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) coupled to mass spectrometry (TG-MS), we obtained data on the thermal behavior and decomposition mechanism for these compounds. We demonstrated that di(1,2,3-triazolyl)-substituted 1,3,5-triazines have an increased thermal stability and have higher values of decomposition onset temperature (220–250 °C) in comparison with tri(1,2,3-triazolyl)-substituted 1,3,5-triazines (180 °C and 160 °C, respectively).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energetic Materials Frontiers
Materials Science-Materials Science (miscellaneous)
CiteScore
6.90
自引率
0.00%
发文量
42
审稿时长
12 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


