电活化血清制备 L(+)- 乳酸
IF 0.9
Q3 Engineering
Surface Engineering and Applied Electrochemistry
Pub Date : 2023-12-14
DOI:10.3103/S1068375523060182
引用次数: 0
摘要
摘要介绍了从不同乳清中提取乳酸的L(+)光学异构体的研究结果:乳酸的初始、发酵和在分离隔膜式电解装置中的浓缩。乳酸的L(+)同分异构体在电解槽的阳极室中得到的速率取决于乳清的浓度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
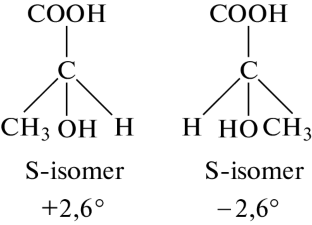
Preparation of L(+)-Lactic Acid upon Electroactivation of Serum
The results from investigations of obtaining the L(+) optical isomer of lactic acid from different types of whey are presented: of the initial, fermented, and concentrated in an electrolysis-type apparatus with a separating diaphragm. The rate of obtaining the L(+) isomer of lactic acid in the anode chamber of the electrolyzer was determined depending on the concentration of whey.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Engineering and Applied Electrochemistry
Engineering-Industrial and Manufacturing Engineering
CiteScore
1.60
自引率
22.20%
发文量
54
期刊介绍:
Surface Engineering and Applied Electrochemistry is a journal that publishes original and review articles on theory and applications of electroerosion and electrochemical methods for the treatment of materials; physical and chemical methods for the preparation of macro-, micro-, and nanomaterials and their properties; electrical processes in engineering, chemistry, and methods for the processing of biological products and food; and application electromagnetic fields in biological systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


