二甲双胍通过调节肠道微生物群促进抗PD-L1的疗效
IF 5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
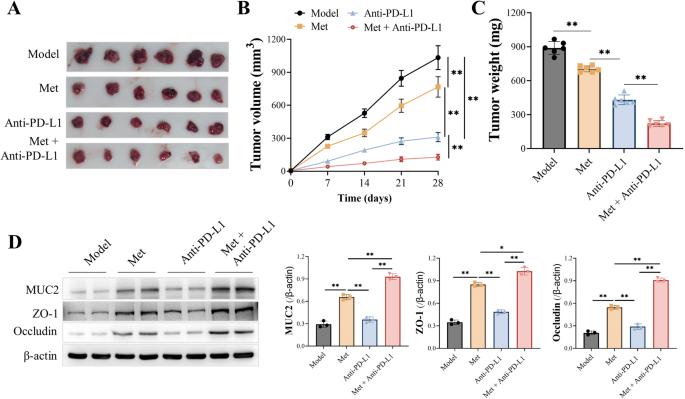
二甲双胍是一种合成的双胍类药物,已被证明对各种人类疾病有有益作用。研究证实,二甲双胍通过调节肠道菌群的组成发挥作用。肠道菌群的组成影响抗pd - l1免疫治疗的疗效。我们认为二甲双胍对肠道菌群的调节可以提高抗pd - l1抗体的治疗效率。在Lewis肺癌C57BL/6J小鼠中,我们发现二甲双胍增强PD-L1抗体的功效主要依赖于肠道菌群的存在,二甲双胍通过调节肠道菌群来增强抗肿瘤免疫,影响肠黏膜的完整性。肠道菌群的抗生素耗竭使PD-L1抗体与二甲双胍的联合药效消失,提示肠道菌群在二甲双胍抗肿瘤作用中的重要作用。抗pd - l1抗体联合二甲双胍可引起CD8 t细胞浸润和IFN-γ表达增加,从而诱发肿瘤坏死。综上所述,二甲双胍可以作为微生态控制器,促进抗肿瘤免疫,提高抗pd - l1抗体的疗效。我们的研究提供了可靠的证据,证明二甲双胍可以与抗pd - l1抗体协同使用,增强抗癌作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Metformin facilitates anti-PD-L1 efficacy through the regulation of intestinal microbiota
Metformin is a synthetic biguanide proven to have beneficial effects against various human diseases. Research has confirmed that metformin exerts its effects by regulating the composition of intestinal microbiota. The composition of intestinal microbiota influences the efficacy of anti-PD-L1 immunotherapy. We assume that the regulation of metformin on intestinal microbiota could enhance the therapeutic efficiency of anti-PD-L1 antibodies. In Lewis lung cancer-bearing C57BL/6J mice, we find that metformin enhances PD-L1 antibody efficacy mainly depending on the existence of gut microbiota, and metformin increases the anti-tumor immunity through modulation of intestinal microbiota and affects the integrity of the intestinal mucosa. Antibiotic depletion of gut microbiota abolished the combination efficacy of PD-L1 antibody and metformin, implying the significance of intestinal microbiota in metformin’s antitumor action. Combining anti-PD-L1 antibody with metformin provoked tumor necrosis by causing increased CD8 T-cell infiltration and IFN-γ expression. In conclusion, metformin could be employed as a microecological controller to prompt antitumor immunity and increase the efficacy of anti-PD-L1 antibodies. Our study provided reliable evidence that metformin could be synergistically used with anti-PD-L1 antibody to enhance the anti-cancer effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


