PES1/FOXM1 异源二聚体抑制卵巢子宫内膜异位症中 TCF21 和 ERβ 的表达
IF 8.5
4区 医学
Q1 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
摘要
转录因子21(TCF21)和雌激素受体β(ERβ,由ESR2编码)在子宫内膜异位基质细胞(ESCs)中高度表达,并对子宫内膜异位症的发病机制起着重要作用。然而,在分子水平上对TCF21和ERβ表达调控的探索仍然有限。在这里,通过生物信息学分析和实验验证,我们发现PES1(又称Pescadillo)是子宫内膜异位症发病过程中的负调控因子,它能下调ESCs中TCF21和ERβ的表达。PES1 的过表达调控了子宫内膜异位症发育过程中的关键生物过程,如侵袭和凋亡。免疫共沉淀试验表明,PES1能与叉头盒M1(FOXM1)形成复合物。进一步的分析表明,在异位病灶中 siPES1 可降低 FOXM1 蛋白的稳定性,减少 FOXM1 与 TCF21 和 ESR2 启动子的结合活性,从而削弱 FOXM1 对 TCF21 和 ERβ 的转录抑制作用。此外,在子宫内膜异位症小鼠模型中,过表达 PES1 能有效减少异位病灶的生长,并抑制 TCF21 和 ERβ 的表达,这为子宫内膜异位症的治疗提供了一种很有前景的策略。总之,我们的研究结果表明,异位病灶中PES1的缺失会通过与FOXM1形成异源二聚体上调ERβ和TCF21的表达,从而导致子宫内膜异位症的进展。此外,靶向 PES1 可作为子宫内膜异位症的一种治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
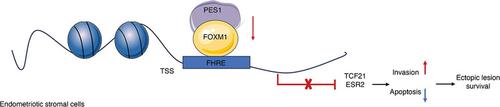
The PES1/FOXM1 heterodimer suppresses TCF21 and ERβ expression in ovarian endometriosis
Transcription factor 21 (TCF21) and estrogen receptor beta (ERβ, encoded by ESR2) are highly expressed in endometriotic stromal cells (ESCs) and contribute to the pathogenesis of endometriosis. However, the exploration of TCF21 and ERβ expression regulation at the molecular level remains limited. Here, by using bioinformatics analysis and experimental verification, we identified PES1, also known as Pescadillo, as a negative regulator in the development of endometriosis that downregulates TCF21 and ERβ expression in ESCs. PES1 overexpression regulated critical biological processes involved in endometriosis development, such as invasion and apoptosis. A coimmunoprecipitation assay showed that PES1 could form a complex with Forkhead box M1 (FOXM1). Further analyses elucidated that siPES1 in ectopic lesions decreased the stability of FOXM1 protein and reduced the binding activities of FOXM1 to TCF21 and ESR2 promoters, thus weakening the transcriptional inhibition of TCF21 and ERβ by FOXM1. Moreover, in an endometriosis mouse model, overexpressing PES1 effectively reduced the growth of ectopic lesions and suppressed TCF21 and ERβ expression, which suggests a promising therapeutic strategy for endometriosis. Collectively, our results indicate that the loss of PES1 in ectopic lesions contributes to endometriosis progression by upregulating ERβ and TCF21 expression through heterodimer formation with FOXM1. Moreover, targeting PES1 could serve as a treatment method for endometriosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

VIEW
Multiple-
CiteScore
12.60
自引率
2.30%
发文量
0
审稿时长
10 weeks
期刊介绍:
View publishes scientific articles studying novel crucial contributions in the areas of Biomaterials and General Chemistry. View features original academic papers which go through peer review by experts in the given subject area.View encourages submissions from the research community where the priority will be on the originality and the practical impact of the reported research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


