通过钴催化的不对称氮杂巴比尔反应合成模块化 α-叔氨基酯
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在α-碳中心含有两个不同碳基取代基的非天然手性α-叔氨基酸广泛存在于生物活性分子中。这种立体刚性支架正日益成为药物发现领域的研究热点。然而,由于立体选择性地构建立体构架的四取代碳中心是一项挑战,因此用于手性 α-叔胺基酸合成的稳健方案仍然匮乏。在此,我们报告了一种由钴催化的对映体选择性氮杂巴比尔反应,它能使酮亚胺与各种未活化的烷基卤化物(包括烷基碘化物、烷基溴化物和烷基氯化物)发生反应,从而生成手性 α-叔氨基酯,该反应具有高度的对映体选择性和优异的官能团耐受性。在这一不对称还原加成方案中,伯、仲和叔有机亲电体均可耐受,这为利用对湿气和空气敏感的有机金属试剂进行对映选择性亲核加成提供了一种补充方法。此外,α-酮酯、胺和卤化烷基的三组分转化代表了羰基的正式不对称脱氧烷基化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
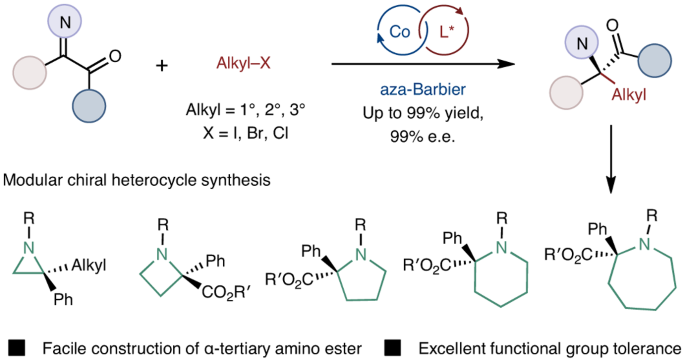
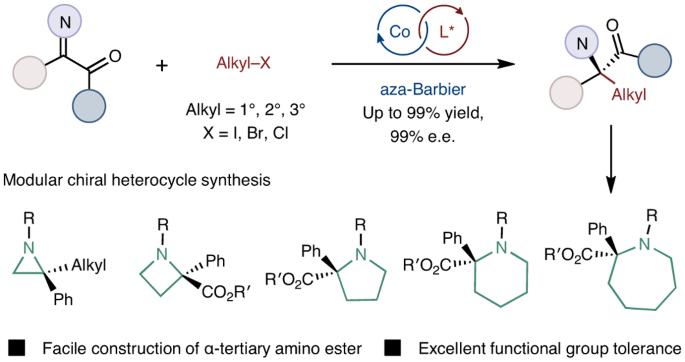
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Unnatural chiral α-tertiary amino acids containing two different carbon-based substituents at the α-carbon centre are widespread in biologically active molecules. This sterically rigid scaffold is becoming a growing research interest in drug discovery. However, a robust protocol for chiral α-tertiary amino acid synthesis remains scarce due to the challenge of stereoselectively constructing sterically encumbered tetrasubstituted stereogenic carbon centres. Herein we report a cobalt-catalysed enantioselective aza-Barbier reaction of ketimines with various unactivated alkyl halides, including alkyl iodides, alkyl bromides and alkyl chlorides, enabling the formation of chiral α-tertiary amino esters with a high level of enantioselectivity and excellent functional group tolerance. Primary, secondary and tertiary organoelectrophiles are all tolerated in this asymmetric reductive addition protocol, which provides a complementary method for the well-exploited enantioselective nucleophilic addition with moisture- and air-sensitive organometallic reagents. Moreover, the three-component transformation of α-ketoester, amine and alkyl halide represents a formal asymmetric deoxygenative alkylamination of the carbonyl group. Robust protocols for the synthesis of chiral α-tertiary amino acids remain scarce due to the challenge of constructing congested tetrasubstituted stereocentres. Now a cobalt-catalysed enantioselective aza-Barbier reaction of ketimines with various unactivated alkyl halides has been developed, forming diverse chiral α-tertiary amino esters with high enantioselectivity and excellent functional group tolerance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


