硫酸镁浸出风化壳淋溶稀土矿中的稀土和铝:浸出剂溶液中铝含量的影响
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
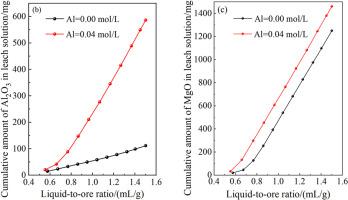
铝是风化壳淋镀稀土矿的主要杂质。稀土的高效浸出和铝的低浸出对WCED-REO的浸出具有重要意义。研究了浸出剂溶液pH、MgSO4浓度和Al3+浓度对硫酸镁柱浸WCED-REO行为的影响。实验数据表明,控制MgSO4浓度为0.15 mol/L,浸出剂溶液pH为2时,随着浸出剂溶液中Al3+浓度的增加,稀土矿中铝的浸出量逐渐减少,说明浸出剂溶液中的Al3+可能作为浸出剂参与了RE3+的离子交换,但稀土的浸出量随Al3+浓度的增加变化不显著。增加MgSO4浓度有利于铝的浸出,当Al3+浓度为0.04 mol/L (Al富集)时,随着MgSO4浓度的增加,稀土矿中Al3+的浸出量逐渐增加。浸出剂溶液的pH值对稀土矿中铝的浸出有显著影响,随着pH值的降低,稀土矿中铝的浸出量逐渐增加。当Al3+浓度为0.04 mol/L (Al富集),浸出剂溶液pH值大于2.0时,浸出剂溶液中的铝可以反吸附到稀土矿上;随着浸出剂溶液pH的增加,Al3+的反吸附量增加。浸出剂溶液的注入速率对稀土和铝的浸出行为影响不大。综上所述,通过调节铝的富集、MgSO4浓度和pH,可以减少Al3+的浸出和MgSO4的消耗。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Leaching of rare earths and aluminum in weathered crust elution-deposited rare earth ore using magnesium sulfate: Effect of aluminum content in leaching agent solution
Aluminum is the main impurity of the weathered crust elution-deposited rare earth ore (WCED-REO). Efficient leaching of rare earths and low leaching of aluminum are of great importance for the leaching of the WCED-REO. The effects of pH, MgSO4 concentration and Al3+ concentration of the leaching agent solution on the column leaching behaviors of WCED-REO using magnesium sulfate were investigated. Experimental data show that controlling the MgSO4 concentration to 0.15 mol/L, pH of the leaching agent solution to 2, the leaching amount of aluminum from the rare earth ore gradually decreases with the increase of Al3+ concentration in the leaching agent solution, indicating that Al3+ in the leaching agent solution may act as leaching agent to participate in the ion exchange of RE3+, but the leaching amounts of rare earths change insignificantly as the Al3+ concentration is increased. Increasing the MgSO4 concentration is beneficial to the leaching of aluminum, and when the Al3+ concentration is 0.04 mol/L (Al accumulation), the amount of Al3+ leached from the rare earth ore increased gradually with increasing the MgSO4 concentration. The pH of the leaching agent solution has a significant influence on the leaching of aluminum in the rare earth ore, and the leaching amount of aluminum from the rare earth ore increases gradually with decreasing the pH. When the Al3+ concentration is 0.04 mol/L (Al accumulation) and the pH of the leaching agent solution is above 2.0, the aluminum in the leaching agent solution can be back-adsorbed onto the rare earth ore, and the amount of the back-adsorbed Al3+ increases with increasing the pH of the leaching agent solution. The injection rate of the leaching agent solution has slight effect on the leaching behavior of rare earths and aluminum. In summary, leaching of Al3+ and consumption of MgSO4 can be reduced by regulating the accumulation of aluminum, MgSO4 concentration and pH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


