血浆蛋白质组中的器官衰老特征跟踪健康和疾病。
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
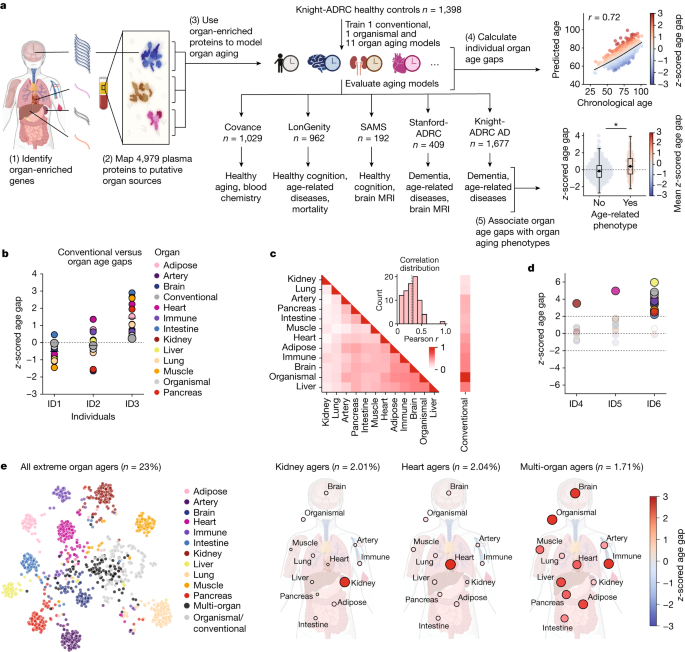
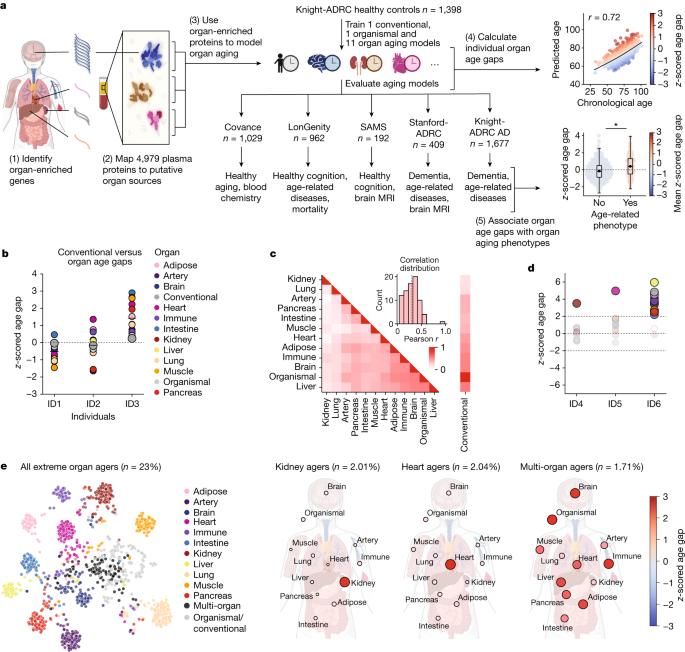
动物研究表明,个体之间以及个体内部器官之间的衰老情况各不相同,但这是否适用于人类以及它对与年龄有关的疾病的影响尚不清楚。我们利用来自特定器官的人类血浆蛋白水平来测量活体个体的器官特异性衰老差异。利用机器学习模型,我们分析了11个主要器官的衰老,并在5个独立的队列中可重复地估计了器官年龄,这些队列包括5676名成年人。我们发现近20%的人在一个器官上表现出强烈的衰老加速,1.7%的人是多器官衰老。器官加速老化会增加20-50%的死亡风险,而器官特异性疾病与这些器官的加速老化有关。我们发现心脏加速老化的个体心力衰竭风险增加250%,大脑和血管加速老化独立于血浆pTau-181预测阿尔茨海默病(AD)的进展,并且与血浆pTau-181一样强(参考文献5),pTau-181是目前最好的AD血液生物标志物。我们的模型将血管钙化、细胞外基质改变和突触蛋白脱落与早期认知能力下降联系起来。我们介绍了一种简单且可解释的方法,利用血浆蛋白质组学数据研究器官衰老,预测疾病和衰老效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Organ aging signatures in the plasma proteome track health and disease
Animal studies show aging varies between individuals as well as between organs within an individual1–4, but whether this is true in humans and its effect on age-related diseases is unknown. We utilized levels of human blood plasma proteins originating from specific organs to measure organ-specific aging differences in living individuals. Using machine learning models, we analysed aging in 11 major organs and estimated organ age reproducibly in five independent cohorts encompassing 5,676 adults across the human lifespan. We discovered nearly 20% of the population show strongly accelerated age in one organ and 1.7% are multi-organ agers. Accelerated organ aging confers 20–50% higher mortality risk, and organ-specific diseases relate to faster aging of those organs. We find individuals with accelerated heart aging have a 250% increased heart failure risk and accelerated brain and vascular aging predict Alzheimer’s disease (AD) progression independently from and as strongly as plasma pTau-181 (ref. 5), the current best blood-based biomarker for AD. Our models link vascular calcification, extracellular matrix alterations and synaptic protein shedding to early cognitive decline. We introduce a simple and interpretable method to study organ aging using plasma proteomics data, predicting diseases and aging effects. Blood plasma protein data was combined with machine learning models for a simple method to determine differences in organ-specific aging; the study provides a basis for the prediction of diseases and aging effects using plasma proteomics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


