光氧化还原辅助酮与芳烯直接a-烷基化反应的研究
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了在有机光催化剂和Brønsted碱的作用下,酮与烯烃的直接a-烷基化反应。结果表明,在2,4,6-三(二苯基氨基)-3,5-二氟苯腈(3DPA2FBN)和LiOtBu的催化量存在下,酮类与苯乙烯类似物的反应在蓝光led照射下顺利进行,可获得中高收率的产物。这种方法构成了环酮和非环酮a功能化的原子经济烷基化过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
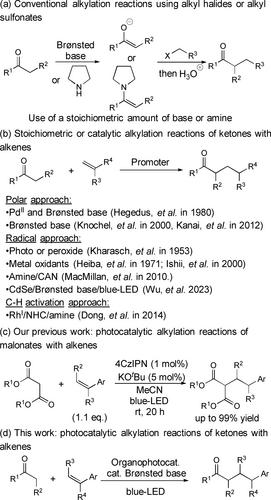
Development of Photoredox-Assisted Direct α-Alkylation Reactions of Ketones with Arylalkenes Using a Catalytic Amount of LiOtBu as a Brønsted Base
Photoinduced direct α-alkylation reactions of ketones with arylalkenes using an organophotocatalyst and a Brønsted base were developed. It was found that the choice of both Brønsted base and photocatalyst was crucial, and in the presence of catalytic amounts of 2,4,6-tris(diphenylamino)-3,5-difluorobenzonitrile (3DPA2FBN) and LiOtBu, the desired reactions of ketones with styrene analogues proceeded smoothly under blue-LED light irradiation to afford the products in moderate to high yields. This method constitutes an atom-economical alkylation process for α-functionalization of both cyclic and acyclic ketones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


