HFIP介导的连续氧化合成c4 -芳基-2-喹诺酮类药物
IF 1.5
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
介绍了一种以丙酰氯和苯胺为原料合成c4芳基化2-喹诺酮类化合物的有效方法。合成过程包括初始产物四氢喹啉和喹啉离子的后续氧化,最终生成所需的喹诺酮类药物。基于对中间体的细致研究和全面的控制实验,提出了一种转化机制。通过对反应机理的深入了解,扩大了反应范围的适用范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
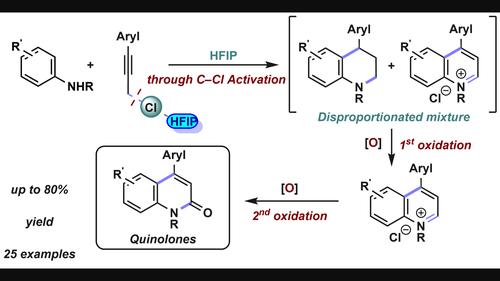
HFIP Mediated Synthesis of C4-Aryl-2-quinolones through Serial Oxidation
A practical, efficient approach for the synthesis of C4-arylated 2-quinolones from propargylic chlorides and anilines has been developed. The synthesis process involves subsequent oxidations of the initial products, tetrahydroquinolines and quinolinium ions, eventually leading to desired quinolones. A mechanism for the transformation is proposed based on a meticulous examination of intermediates and comprehensive control experiments. With a thorough understanding of the reaction mechanism, the applicability of the reaction scope is expanded.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Helvetica Chimica Acta
化学-化学综合
CiteScore
3.00
自引率
0.00%
发文量
60
审稿时长
2.3 months
期刊介绍:
Helvetica Chimica Acta, founded by the Swiss Chemical Society in 1917, is a monthly multidisciplinary journal dedicated to the dissemination of knowledge in all disciplines of chemistry (organic, inorganic, physical, technical, theoretical and analytical chemistry) as well as research at the interface with other sciences, where molecular aspects are key to the findings. Helvetica Chimica Acta is committed to the publication of original, high quality papers at the frontier of scientific research. All contributions will be peer reviewed with the highest possible standards and published within 3 months of receipt, with no restriction on the length of the papers and in full color.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


