立体化学编辑通过耦合的外显异构和光学分辨率,以可持续地制造一个关键的Adagrasib构建块
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
对不需要的哌嗪立体异构体进行外异构化和再循环,使手性萘酰胺构建块的合成更加可持续、简洁和高产。因此,非选择性键形成类似于正式的对映选择性aza-Michael反应。该策略缩短了开发时间,消除了氰化钠的处理,消除了两次化学键形成操作,并将工艺质量强度降低了近3倍。总产量由34%提高到74%。选择广泛可用的商品化学品作为构建模块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
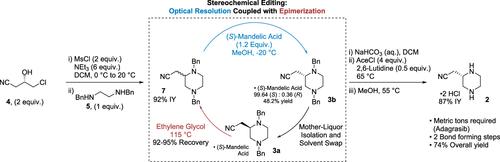
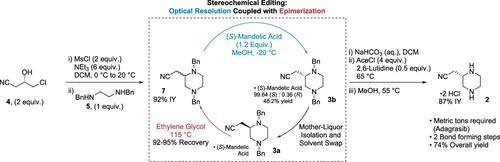
Stereochemical Editing via Coupled Epimerization and Optical Resolution to Sustainably Manufacture a Key Adagrasib Building Block
Epimerization and recycling of an unwanted piperazine stereoisomer enabled a more sustainable, concise, and high-yielding synthesis of a chiral adagrasib building block. The unselective bond formation thus resembled a formal enantioselective aza-Michael reaction. This strategy shortened development timelines, eliminated the handling of sodium cyanide, removed two chemical bond-forming operations, and decreased the process mass intensity nearly 3-fold. The overall yield was improved from 34 to 74%. Widely available commodity chemicals were selected as building blocks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


