减数分裂重组机制的分化与保存。
IF 39.1
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
有性生殖的真核生物在减数分裂过程中利用同源染色体之间的重组促进染色体分离。减数分裂重组在其大致范围内几乎是普遍保守的,但在不同的分类群之间,具体的分子细节往往差别很大,构成重组机制的蛋白质显示出实质性的序列可变性。由于最近基因组资源的增加和蛋白质结构预测的进展,这种变异的程度正变得越来越清楚。我们讨论功能保护和快速进化变化之间的紧张关系,重点关注减数分裂DNA双链断裂形成和修复所需的蛋白质。我们强调了不同时间尺度上的系统发育关系,并提出这种显著的进化可塑性是减数分裂重组的基本特性,它塑造了我们对生殖生物学分子机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
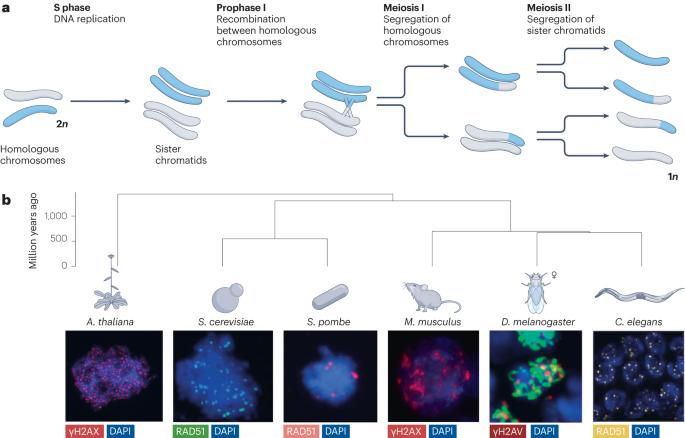
Divergence and conservation of the meiotic recombination machinery
Sexually reproducing eukaryotes use recombination between homologous chromosomes to promote chromosome segregation during meiosis. Meiotic recombination is almost universally conserved in its broad strokes, but specific molecular details often differ considerably between taxa, and the proteins that constitute the recombination machinery show substantial sequence variability. The extent of this variation is becoming increasingly clear because of recent increases in genomic resources and advances in protein structure prediction. We discuss the tension between functional conservation and rapid evolutionary change with a focus on the proteins that are required for the formation and repair of meiotic DNA double-strand breaks. We highlight phylogenetic relationships on different time scales and propose that this remarkable evolutionary plasticity is a fundamental property of meiotic recombination that shapes our understanding of molecular mechanisms in reproductive biology. In this Review, the authors describe the evolutionary conservation and divergence of the meiotic recombination machinery, focusing on proteins that are required for meiotic double-strand break formation, double-strand break repair via homologous recombination and the formation of crossover and non-crossover recombinant DNA molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Reviews Genetics
生物-遗传学
CiteScore
57.40
自引率
0.50%
发文量
113
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Genetics, our goal is to be the leading source of reviews and commentaries for the scientific communities we serve. We are dedicated to publishing authoritative articles that are easily accessible to our readers. We believe in enhancing our articles with clear and understandable figures, tables, and other display items. Our aim is to provide an unparalleled service to authors, referees, and readers, and we are committed to maximizing the usefulness and impact of each article we publish.
Within our journal, we publish a range of content including Research Highlights, Comments, Reviews, and Perspectives that are relevant to geneticists and genomicists. With our broad scope, we ensure that the articles we publish reach the widest possible audience.
As part of the Nature Reviews portfolio of journals, we strive to uphold the high standards and reputation associated with this esteemed collection of publications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


