体系中的固溶体为NaMgAl(SO4)3 - kmgal (SO4)3
摘要
在20 mol / l的合成范围内合成了NaMgAl(SO4)3 - kmgal (SO4)3% increments from pure Na to pure K compounds. We investigated them by Powder X-Ray diffraction, 23Na, and 27Al Nuclear Magnetic Resonance spectroscopy. The results confirm NaMgAl(SO4)3 as a unique phase identical to a presumed new mineral found in the fumaroles of Eldfell and Hekla volcanoes in Iceland. It tolerates less than 10 mol% K substitution for Na. There exists a compositional gap to approximately Na0.65K0.35MgAl(SO4)3 from where a solid solution extends to KMgAl(SO4)3. The mineral koryakite [NaKMg2Al2(SO4)6] is a member of the latter solid solution series. The crystal structures of all (Na,K)MgAl(SO4)3 phases are akin to NASICON (NA Super Ionic CONductor). NaMgAl(SO4)3 has \(R\overline{3}c\) symmetry and a disordered distribution of Mg and Al among the octahedral sites with only one unique site for the alkali atom. The members of the solid solution have \(R\overline{3}\) symmetry with ordered Mg–Al distribution and two unique alkali sites with different preferences for Na and K. In the crystal structure, the coordination of Na and/or K is trigonal antiprismatic, and these share bases with two octahedral Mg (Na) or Al (K) coordinations. These polyhedra are arranged in columns parallel to [001] and interconnected by SO4 tetrahedral groups. The alkali atoms from a column lie in the same (001) layers as the octahedrally coordinated atoms from the three neighboring rows. On the same level, parallel to (001), there are gaps in the other three neighboring columns forming channels containing Na+ or K+ ions.
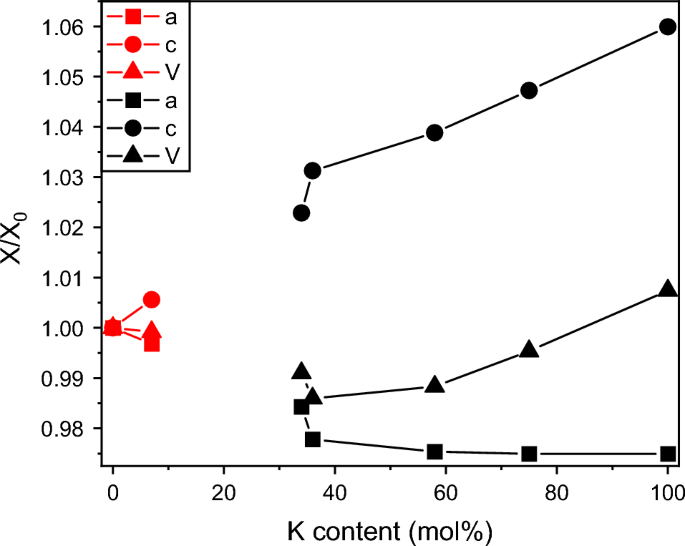
We synthesized six samples in the compositional field NaMgAl(SO4)3–KMgAl(SO4)3 in 20 mol% increments from pure Na to pure K compounds. We investigated them by Powder X-Ray diffraction, 23Na, and 27Al Nuclear Magnetic Resonance spectroscopy. The results confirm NaMgAl(SO4)3 as a unique phase identical to a presumed new mineral found in the fumaroles of Eldfell and Hekla volcanoes in Iceland. It tolerates less than 10 mol% K substitution for Na. There exists a compositional gap to approximately Na0.65K0.35MgAl(SO4)3 from where a solid solution extends to KMgAl(SO4)3. The mineral koryakite [NaKMg2Al2(SO4)6] is a member of the latter solid solution series. The crystal structures of all (Na,K)MgAl(SO4)3 phases are akin to NASICON (NA Super Ionic CONductor). NaMgAl(SO4)3 has \(R\overline{3}c\) symmetry and a disordered distribution of Mg and Al among the octahedral sites with only one unique site for the alkali atom. The members of the solid solution have \(R\overline{3}\) symmetry with ordered Mg–Al distribution and two unique alkali sites with different preferences for Na and K. In the crystal structure, the coordination of Na and/or K is trigonal antiprismatic, and these share bases with two octahedral Mg (Na) or Al (K) coordinations. These polyhedra are arranged in columns parallel to [001] and interconnected by SO4 tetrahedral groups. The alkali atoms from a column lie in the same (001) layers as the octahedrally coordinated atoms from the three neighboring rows. On the same level, parallel to (001), there are gaps in the other three neighboring columns forming channels containing Na+ or K+ ions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


