铜催化双氟烯在温和条件下立体选择性脱氟烷基化或芳基化生成单氟烯
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这里,我们展示了通过铜催化的脱氟烷基化或宝石二氟烯的脱氟芳基化形成单氟烯,使用价格合理且易于获得的格氏试剂。此外,烷基锂或烷基锌试剂在这些转化中被证明是成功的。在温和的条件下,单氟烯可转化为氟化烯烃化合物或非氟化炔化合物。最初的机理研究表明,该反应可能从有机铜试剂开始,并经历一个快速的β-F消除步骤。虽然目前的对映体过量是低的,手性配体可以促进该反应的不对称转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
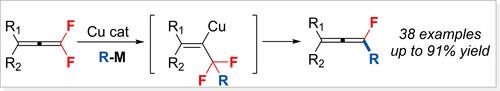
Copper‐Catalyzed Stereoselective Defluoroalkylation or ‐arylation of gem‐Difluoroallenes To Form Monofluoroallenes under Mild Conditions
Herein, we demonstrate the formation of monofluoroallenes through copper‐catalyzed defluoroalkylation or defluoroarylation of gem‐difluoroallenes using affordable and readily available Grignard reagents. Additionally, alkyl lithium or alkyl zinc reagents proved successful in these transformations. Under mild conditions, monofluoroallenes can be converted into fluorinated olefinic compounds or non‐fluorinated alkyne compounds. Initial mechanistic studies suggest that the reaction may start with an organocopper reagent and undergo a rapid β‐F elimination step. Although the current enantiomeric excess is low, chiral ligands could facilitate the asymmetric transformation of this reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


