阴离子纳米塑料污染物促进帕金森病相关α-突触核蛋白聚集。
IF 3.5
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
最近的研究发现,环境中的纳米塑料污染水平不断上升。在这里,我们发现阴离子纳米塑料污染物通过与α-突触核蛋白的两亲性和非淀粉样成分(NAC)结构域的高亲和力相互作用,有力地沉淀α-突触核蛋白原纤维的形成和繁殖。纳米塑料可以通过网格蛋白依赖的内吞作用在神经元中内化,引起轻度溶酶体损伤,减缓聚集的α-突触核蛋白的降解。在小鼠中,纳米塑料与α-突触核蛋白原纤维结合,加剧了α-突触核蛋白病理在相互关联的大脑脆弱区域的传播,包括在黑质多巴胺能神经元中强烈诱导α-突触核蛋白包涵体。这些结果为进一步探索纳米塑料污染与帕金森病和相关痴呆相关的α-突触核蛋白聚集之间的潜在联系提供了线索。本文章由计算机程序翻译,如有差异,请以英文原文为准。
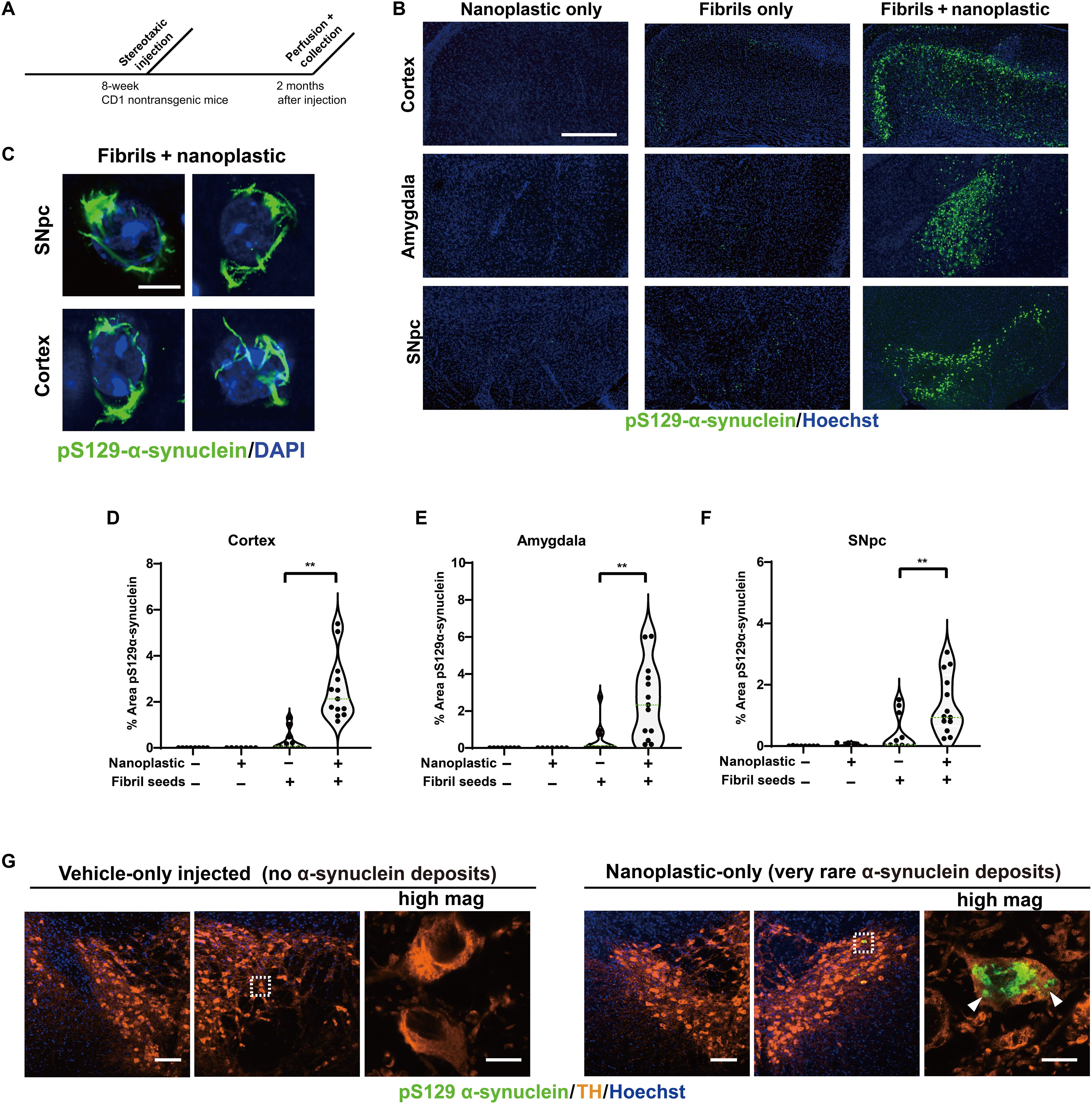
Anionic nanoplastic contaminants promote Parkinson’s disease–associated α-synuclein aggregation
Recent studies have identified increasing levels of nanoplastic pollution in the environment. Here, we find that anionic nanoplastic contaminants potently precipitate the formation and propagation of α-synuclein protein fibrils through a high-affinity interaction with the amphipathic and non-amyloid component (NAC) domains in α-synuclein. Nanoplastics can internalize in neurons through clathrin-dependent endocytosis, causing a mild lysosomal impairment that slows the degradation of aggregated α-synuclein. In mice, nanoplastics combine with α-synuclein fibrils to exacerbate the spread of α-synuclein pathology across interconnected vulnerable brain regions, including the strong induction of α-synuclein inclusions in dopaminergic neurons in the substantia nigra. These results highlight a potential link for further exploration between nanoplastic pollution and α-synuclein aggregation associated with Parkinson’s disease and related dementias.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Chemical Biology
生物-生化与分子生物学
CiteScore
7.50
自引率
5.00%
发文量
353
审稿时长
3.3 months
期刊介绍:
ACS Chemical Biology provides an international forum for the rapid communication of research that broadly embraces the interface between chemistry and biology.
The journal also serves as a forum to facilitate the communication between biologists and chemists that will translate into new research opportunities and discoveries. Results will be published in which molecular reasoning has been used to probe questions through in vitro investigations, cell biological methods, or organismic studies.
We welcome mechanistic studies on proteins, nucleic acids, sugars, lipids, and nonbiological polymers. The journal serves a large scientific community, exploring cellular function from both chemical and biological perspectives. It is understood that submitted work is based upon original results and has not been published previously.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


