同时时空转录组学和显微镜观察枯草芽孢杆菌群发育揭示了跨代合作。
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
微生物群落的发展是一个复杂的多尺度现象,具有广泛的生物医学和生态学意义。由于缺乏对基因表达和细胞行为在空间和时间上的同步测量,生物和物理过程如何决定微生物群落中出现的空间结构仍然知之甚少。在这里,我们将活细胞显微镜与机械臂结合起来进行时空采样,这使我们能够同时获得枯草芽孢杆菌群体发育过程中的表型成像数据和时空转录组。时空基因表达模式的定量表征揭示了与细胞和集体特性以及表型亚群的相关性。通过将这些数据与时空代谢组测量相结合,我们发现了一种时空交叉取食机制,促进了群体的发展:在迁徙过程中,较早的一代沉积代谢物,这些代谢物被跨越同一地点的后代消耗。这些结果强调了在细菌群落中出现表型亚群及其相互作用过程中时空效应的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
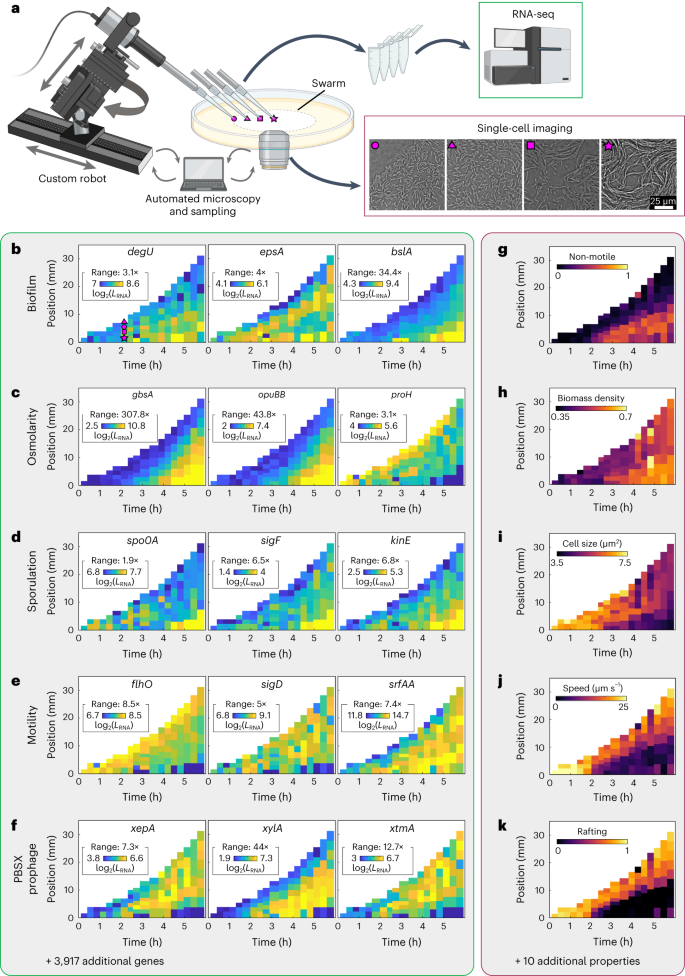
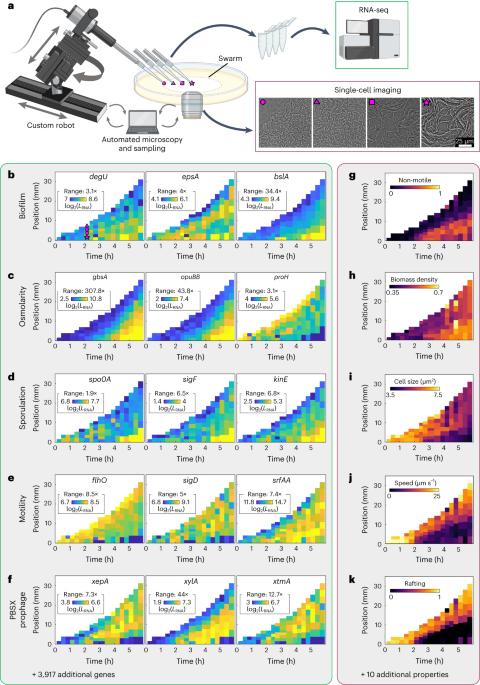
Simultaneous spatiotemporal transcriptomics and microscopy of Bacillus subtilis swarm development reveal cooperation across generations
Development of microbial communities is a complex multiscale phenomenon with wide-ranging biomedical and ecological implications. How biological and physical processes determine emergent spatial structures in microbial communities remains poorly understood due to a lack of simultaneous measurements of gene expression and cellular behaviour in space and time. Here we combined live-cell microscopy with a robotic arm for spatiotemporal sampling, which enabled us to simultaneously acquire phenotypic imaging data and spatiotemporal transcriptomes during Bacillus subtilis swarm development. Quantitative characterization of the spatiotemporal gene expression patterns revealed correlations with cellular and collective properties, and phenotypic subpopulations. By integrating these data with spatiotemporal metabolome measurements, we discovered a spatiotemporal cross-feeding mechanism fuelling swarm development: during their migration, earlier generations deposit metabolites which are consumed by later generations that swarm across the same location. These results highlight the importance of spatiotemporal effects during the emergence of phenotypic subpopulations and their interactions in bacterial communities. Spatiotemporal transcriptomes during multicellular development of Bacillus subtilis swarms reveal supra-generational cooperation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


