通过酶促氢原子转移对氮扎芳烃进行远程立体控制。
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
传统上,实现与扎扎芳烃不对称催化的策略缺乏实现远程立体控制,这将大大提高具有远程手性中心的不同扎扎芳烃的可及性。实现远程立体控制优越的对映体选择性的主要障碍是固有的刚性氮杂环结构。本文介绍了一种烯还原酶系统,该系统能够通过手性氢原子转移机制调节氮杂芳烃上远端碳中心自由基的对映选择性。这一光酶过程有效地从氮原子中引导位于6个以上化学键(或6个以上Å)的前手性自由基中心,从而使生产具有远端γ-立体中心的广泛的扎扎芳烃成为可能。我们综合计算和实验研究的结果强调了关键氨基酸残基的氢键和空间效应对于实现如此高的立体选择性是重要的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
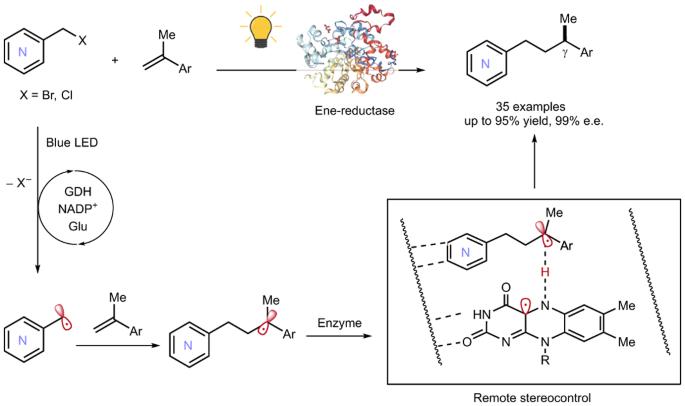
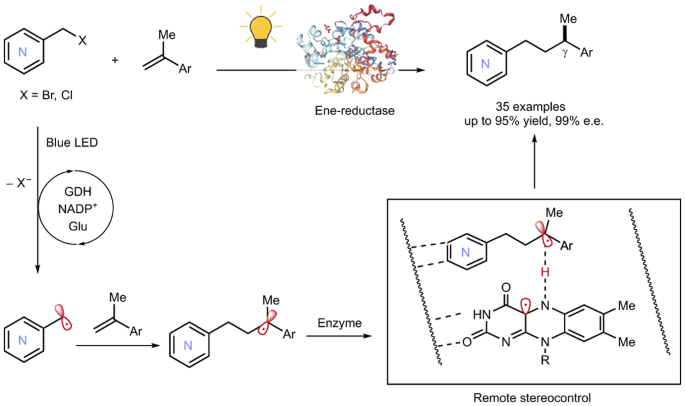
Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
Strategies for achieving asymmetric catalysis with azaarenes have traditionally fallen short of accomplishing remote stereocontrol, which would greatly enhance accessibility to distinct azaarenes with remote chiral centres. The primary obstacle to achieving superior enantioselectivity for remote stereocontrol has been the inherent rigidity of the azaarene ring structure. Here we introduce an ene-reductase system capable of modulating the enantioselectivity of remote carbon-centred radicals on azaarenes through a mechanism of chiral hydrogen atom transfer. This photoenzymatic process effectively directs prochiral radical centres located more than six chemical bonds, or over 6 Å, from the nitrogen atom in azaarenes, thereby enabling the production of a broad array of azaarenes possessing a remote γ-stereocentre. Results from our integrated computational and experimental investigations underscore that the hydrogen bonding and steric effects of key amino acid residues are important for achieving such high stereoselectivities. The inherent rigidity of the azaarene ring structure has made it challenging to achieve remote stereocontrol through asymmetric catalysis on these substrates. Now, through a photoenzymatic process, an ene-reductase system facilitates the production of diverse azaarenes with distant γ-stereocentres, highlighting the potential of biocatalysts for stereoselectivity at remote sites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


