铜催化对映体自由基 C(sp3)-N 交叉偶联以获得手性 α-氨基-β-内酰胺
引用次数: 0
摘要
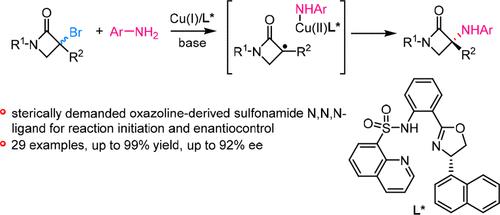
在温和的热反应条件下,开发了一种铜催化的外消旋叔α-溴-β-内酰胺与芳香胺的对映体自由基 C(sp3)-N 交叉偶联反应。使用具有立体需求的噁唑啉衍生磺酰胺 N,N,N-配体对于反应的启动和有效区分氮杂环丁酮衍生环状烷基自由基至关重要。该策略为获得手性 α-氨基-β-内酰胺(许多生物活性分子中的重要结构基团)提供了一种极具吸引力的方法。初步的机理研究支持由 L*Cu(I)-amido 复合物和 α-bromo-β- 内酰胺形成氮杂环丁酮衍生烷基自由基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Copper-Catalyzed Enantioconvergent Radical C(sp3)–N Cross-Coupling to Access Chiral α-Amino-β-lactams
A copper-catalyzed enantioconvergent radical C(sp3)–N cross-coupling of racemic tertiary α-bromo-β-lactams with aromatic amines is developed under mild thermal reaction conditions. The use of a sterically demanded oxazoline-derived sulfonamide N,N,N-ligand is crucial for the reaction initiation and effective enantio-discrimination of the azetidinone-derived cyclic alkyl radicals. The strategy provides an attractive approach to access chiral α-amino-β-lactams, an important structural motif in many biologically active molecules. Preliminary mechanistic studies support the formation of azetidinone-derived alkyl radicals from the L*Cu(I)-amido complex and α-bromo-β-lactams.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Precision Chemistry
精密化学技术-
CiteScore
0.80
自引率
0.00%
发文量
0
期刊介绍:
Chemical research focused on precision enables more controllable predictable and accurate outcomes which in turn drive innovation in measurement science sustainable materials information materials personalized medicines energy environmental science and countless other fields requiring chemical insights.Precision Chemistry provides a unique and highly focused publishing venue for fundamental applied and interdisciplinary research aiming to achieve precision calculation design synthesis manipulation measurement and manufacturing. It is committed to bringing together researchers from across the chemical sciences and the related scientific areas to showcase original research and critical reviews of exceptional quality significance and interest to the broad chemistry and scientific community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


