通过还原胺化优化立体选择性和可扩展的五元环反式-β-氨基酸构建模块的合成
IF 1.7
4区 化学
引用次数: 0
摘要
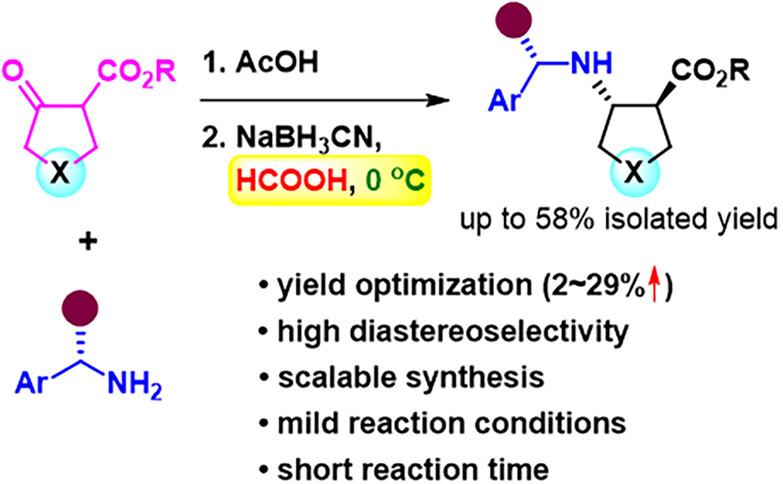
我们介绍了一种立体选择性合成五元脂环和杂环反式-β-氨基酸衍生物的优化方法。该方法涉及使用手性辅助胺对β-酮酯进行还原胺化,以甲酸作为促进剂,在温和的条件下进行快速、非对映选择性还原。我们的方法显著提高了分离产率,并可规模化生产反式-2-氨基环戊烷羧酸(反式-ACPC)、4-氨基吡咯烷-3-羧酸(反式-APC)、4-氨基四氢呋喃-3-羧酸(反式-ATFC)和 4-氨基四氢噻吩-3-羧酸(反式-ATC)构建模块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimized stereoselective and scalable synthesis of five-membered cyclic trans-β-amino acid building blocks via reductive amination
We present an optimized method for the stereoselective synthesis of five-membered alicyclic and heterocyclic trans-β-amino acid derivatives. The process involves a reductive amination of β-keto esters using chiral auxiliary amines, with formic acid acting as a facilitator for rapid, diastereoselective reductions under gentle conditions. Our approach notably enhances isolated yields and permits the scalable production of trans-2-aminocyclopentanecarboxylic acid (trans-ACPC), 4-aminopyrrolidine-3-carboxylic acid (trans-APC), 4-aminotetrahydrofuran-3-carboxylic acid (trans-ATFC), and 4-aminotetrahydrothiophene-3-carboxylic acid (trans-ATTC) building blocks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bulletin of the Korean Chemical Society
Chemistry-General Chemistry
自引率
23.50%
发文量
182
期刊介绍:
The Bulletin of the Korean Chemical Society is an official research journal of the Korean Chemical Society. It was founded in 1980 and reaches out to the chemical community worldwide. It is strictly peer-reviewed and welcomes Accounts, Communications, Articles, and Notes written in English. The scope of the journal covers all major areas of chemistry: analytical chemistry, electrochemistry, industrial chemistry, inorganic chemistry, life-science chemistry, macromolecular chemistry, organic synthesis, non-synthetic organic chemistry, physical chemistry, and materials chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


