四唑融合咪唑并吡啶的设计、合成和生物学研究
IF 5.5
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
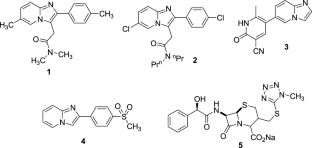
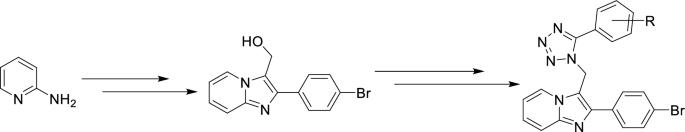
利用以多柔比星为标准药物的 MTT 还原试验,开发了新的四唑融合咪唑吡啶衍生物 (12a-j),以开发其对癌症细胞系--MCF7、A549 和 MDA-MB-231 的细胞毒性活性。化合物 12 h 和 12j 对 A549 细胞株具有很强的抗癌活性,其 IC50 值分别为 1.44 µM 和 1.33 µM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis and biological studies of tetrazole fused imidazopyridines
New tetrazole fused imidazopyridine derivatives (12a–j) were developed to exploit their cytotoxic activity towards cancer cell lines-MCF7, A549, and MDA-MB-231, utilizing MTT reduction assay with doxorubicin as standard drug. The compounds 12 h and 12j demonstrated strong anticancer activity bearing IC50 values 1.44 µM and 1.33 µM against A549 cell line.
Graphical abstract
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon Letters
CHEMISTRY, MULTIDISCIPLINARY-MATERIALS SCIENCE, MULTIDISCIPLINARY
CiteScore
7.30
自引率
20.00%
发文量
118
期刊介绍:
Carbon Letters aims to be a comprehensive journal with complete coverage of carbon materials and carbon-rich molecules. These materials range from, but are not limited to, diamond and graphite through chars, semicokes, mesophase substances, carbon fibers, carbon nanotubes, graphenes, carbon blacks, activated carbons, pyrolytic carbons, glass-like carbons, etc. Papers on the secondary production of new carbon and composite materials from the above mentioned various carbons are within the scope of the journal. Papers on organic substances, including coals, will be considered only if the research has close relation to the resulting carbon materials. Carbon Letters also seeks to keep abreast of new developments in their specialist fields and to unite in finding alternative energy solutions to current issues such as the greenhouse effect and the depletion of the ozone layer. The renewable energy basics, energy storage and conversion, solar energy, wind energy, water energy, nuclear energy, biomass energy, hydrogen production technology, and other clean energy technologies are also within the scope of the journal. Carbon Letters invites original reports of fundamental research in all branches of the theory and practice of carbon science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


