利用装有金刚石电极的流动反应器对异丙烯进行选择性电氧化
Q2 Materials Science
Current Research in Green and Sustainable Chemistry
Pub Date : 2023-01-01
DOI:10.1016/j.crgsc.2023.100378
引用次数: 0
摘要
虽然苯基C(sp3)−H键的氧化是合成生物活性化合物的重要过程,但这些反应通常需要危险的试剂、催化剂和恶劣的条件。由于电有机合成利用电本身作为试剂,因此即使在环境条件下,反应也能进行得很好。在此,我们报道了在电化学流动反应器中对异丙烯的选择性氧化。在最佳电荷量和最佳电流密度条件下,选择性地制备了Cumyl醇。电化学流动反应器抑制了异丙醇过度氧化生成苯乙酮,实现了异丙烯选择性氧化生成异丙醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
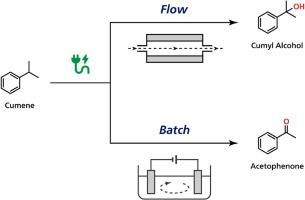
Selective electro-oxidation of cumene utilizing a flow reactor equipped with diamond electrodes
Although oxidation of benzylic C(sp3)−H bonds is an important process for synthesizing biologically active compounds, the reactions generically require hazardous reagents, catalysts, and harsh conditions. Since electro-organic synthesis utilizes electricity itself as a reagent, reactions can proceed well even under ambient conditions. Herein, we report a selective oxidation of cumene in an electrochemical flow reactor. Cumyl alcohol was selectively obtained under optimum amount of charge and current density. The selective oxidation of cumene to cumyl alcohol was achieved because overoxidation of cumyl alcohol into acetophenone was suppressed by the electrochemical flow reactor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Green and Sustainable Chemistry
Materials Science-Materials Chemistry
CiteScore
11.20
自引率
0.00%
发文量
116
审稿时长
78 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


