未活化烯烃的不对称 C-C 交叉偶联
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
几十年来,金属催化的交叉偶联一直在现代化学合成中发挥着核心作用。未活化烯烃(包括轻烯烃)在石油工业中的生产规模巨大,是制备药物、农用化学品和材料的理想起始原料。然而,非活化烯烃的对映选择性交叉偶联仍然是一个具有挑战性的长期目标。在此,我们报告了未活化烯烃与芳基(或烯基)三酸盐和有机金属(或还原剂)的高对映和区域选择性三组分交叉偶联,从而在镍催化下构建出多种 C sp3 立体中心。具体来说,选择性碳镍化和与亲核物的原位捕获能够以无定向基团的方式对未活化的烯烃进行高效的氢官能化和二官能化。带有笨重的 C2 对称手性 N- 杂环碳烯配体的镍催化剂对于实现高反应性和高选择性至关重要。这一策略为快速将原料烯烃升级为各种高附加值分子提供了一个通用、模块化和多样化的平台,并有望激励开发其他具有挑战性的对映选择性烯烃交叉偶联反应。未活化烯烃的对映选择性 C-C 交叉偶联具有挑战性。现在,带有笨重的 C2 对称手性 N- 杂环碳烯配体的镍催化剂能够指导未活化烯烃与芳基或烯基三酸酯和亲核物进行无基团不对称交叉偶联,生成 C sp3 立体中心。本文章由计算机程序翻译,如有差异,请以英文原文为准。
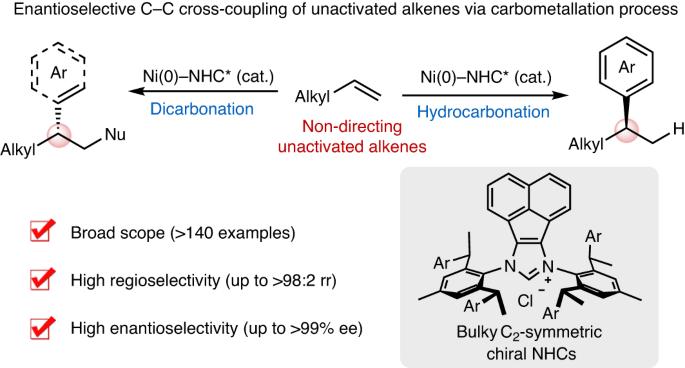
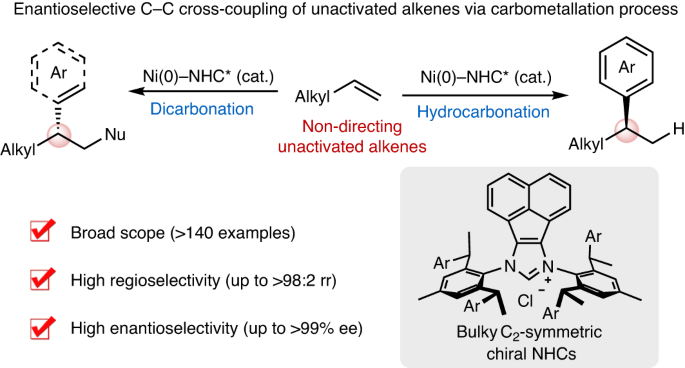
Enantioselective C–C cross-coupling of unactivated alkenes
Metal-catalysed cross-coupling has played a central role in modern chemical synthesis for decades. Unactivated alkenes, including light olefins, are manufactured on enormous scale in the petroleum industry and represent ideal starting materials for the preparation of pharmaceuticals, agrochemicals and materials. However, enantioselective cross-coupling of unactivated alkenes remains a challenging and long-standing goal. Here we report a highly enantio- and regioselective three-component cross-coupling of unactivated alkenes with aryl (or alkenyl) triflates and organometallics (or reductant) to construct diverse C sp3 stereocentres by nickel catalysis. Specifically, selective carbonickelation and in situ trapping with nucleophiles enable efficient hydrofunctionalization and dicarbofunctionalization of unactivated alkenes in a directing group-free manner. Nickel catalysts bearing bulky C2-symmetric chiral N-heterocyclic carbene ligands were crucial for attaining high reactivity and selectivity. This strategy offers a general, modular and divergent platform for rapidly upgrading feedstock alkenes to various value-added molecules and is expected to inspire the development of other challenging enantioselective alkene cross-couplings. Enantioselective C–C cross-coupling of unactivated alkenes is challenging. Now, nickel catalysts bearing bulky C2-symmetric chiral N-heterocyclic carbene ligands enable directing group-free asymmetric cross-coupling of unactivated alkenes with aryl or alkenyl triflates and nucleophiles, generating C sp3 stereocentres.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


