将 2-((二苯氧基磷酰)氧基)-2-芳基乙酸烷基酯转化为 α,α-二芳基酯的 Friedel-Crafts 型芳基化策略
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
据报道,一种芳基化策略可将 2-((二苯氧基磷酰)氧基)-2-芳基乙酸烷基酯转化为 α,α-二酯。当起始有机磷酸盐的芳基环上不带对位烷氧基时,TfOH 可以促进这种转化,但当存在这种基团时,则不需要任何添加剂。这些 2-((二苯氧基磷酰)氧基)-2-芳基乙酸烷基酯很容易从磷酸二苯酯插入芳基重氮乙酸酯中获得。本文章由计算机程序翻译,如有差异,请以英文原文为准。
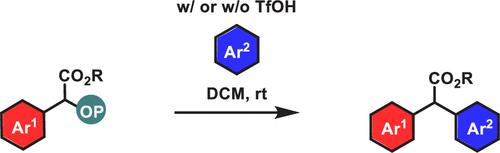
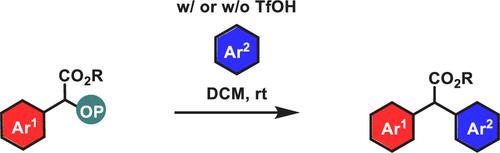
Friedel–Crafts-Type Arylation Strategy for the Conversion of Alkyl 2-((Diphenoxyphosphoryl)oxy)-2-arylacetates to α,α-Diaryl Esters
An arylation strategy allowing the conversion of alkyl 2-((diphenoxyphosphoryl)oxy)-2-arylacetates to α,α-diaryl esters is reported. This transformation can be promoted by TfOH when the starting organic phosphates do not carry para-alkoxy groups on their aryl rings, but it does not require any additives when such groups are present. These alkyl 2-((diphenoxyphosphoryl)oxy)-2-arylacetates can be readily accessed from the insertion of diphenyl phosphate into aryldiazoacetates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


