五味子提取物在正常大鼠体内的药动学研究及LC-MS/MS同时定量
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景:五味子被广泛使用。它有许多药理活性。木质素素是五味子的主要有效成分,包括五味子素A、五味子素A、五味子素B、五味子酚、五味子素E、五味子素H、五味子素J、五味子素N等。目的:建立液相色谱-串联质谱(LC-MS/MS)同时定量测定正常大鼠五味子中木脂素含量的方法。方法:以硝苯地平为内标,在Agela Venusil C18 Plus (4.6*100mm, 3μm)上进行色谱分离。以0.1% (v/v)甲酸水溶液为流动相A, 0.1% (v/v)甲酸甲醇溶液为流动相B进行梯度洗脱。流速0.8 mL/min。采用多反应监测(MRM)模式,正电喷雾电离对分析物进行检测。结果:五味子甲素、五味子甲素B、五味子酚、五味子甲素E、五味子甲素H、五味子甲素N浓度为0.5 ~ 200 ng/ml,五味子甲素A浓度为10 ~ 200 ng/ml,五味子甲素j浓度为5 ~ 200 ng/ml,五味子甲素的日内变异系数(CVs)在6.70%(3.44%)~ 11.66%(10.38%)范围内具有可靠的标度。8种木脂素的日间和日间准确度在95.70%(93.89%)~ 104.59%(106.13%)之间。在稳定性试验中,任何木脂素都没有发生显著变化。结论:所建立的方法可用于五味子提取物在正常大鼠体内的药动学研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
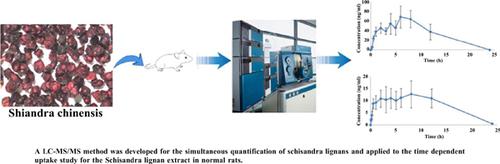
Pharmacokinetics Study and Simultaneous Quantification of Eight Schisandra Lignans in Normal Rats by LC-MS/MS after Oral Administration of Schisandra Lignan Extract
Background: Schisandra chinensis has been widely used. It has many pharmacological activities. Lignans, including schizandrol A, schizandrin A, schisandrin B, schisanhenol, gomisin E, gomisin H, gomisin J, gomisin N, etc., are the major active ingredients of Schisandra chinensis. Objective: In the present study, the liquid chromatography-tandem mass spectrometric (LC-MS/MS) method was developed for the simultaneous quantification of Schisandra lignans in normal rats. Methods: Nifedipine was used as an internal standard, and chromatographic separation was achieved on Agela Venusil C18 Plus (4.6*100mm, 3μm). Aqueous solution containing 0.1% (v/v) formic acid was used as the mobile phase A, and methanol solution containing 0.1% (v/v) formic acid was used as the mobile phase B for gradient elution. The flow rate was 0.8 mL/min. Multiple reaction monitoring (MRM) mode with positive electrospray ionization was used to detect the analytes. Results: The calibration curves provided reliable responses at concentrations of 0.5-200 ng/ml for schizandrin A, schisandrin B, schisanhenol, gomisin E, gomisin H, gomisin N, concentrations of 10-200 ng/ml for schizandrol A, and concentrations of 5-200 ng/ml for gomisin J. The inter- and intra-day coefficients of variations (CVs) for the precision ranged from 6.70% (3.44%) to 11.66% (10.38%). The inter- and intra-day accuracies of eight lignans ranged from 95.70% (93.89%) to 104.59% (106.13%). No significant variation of any of the lignans occurred in the stability tests. Conclusion: The established method can be successfully applied to the pharmacokinetic study of the Schisandra lignan extract in normal rats.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


