针对脂蛋白(a)的新型 siRNA 的临床前开发和 1 期试验
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 94
摘要
有确凿证据表明,脂蛋白(a)(Lp(a))在心血管疾病中起着因果作用。目前还没有直接针对脂蛋白(a)的药物疗法可用于临床。在此,我们报告了奥帕西兰的发现和开发过程,它是一种合成的、双链、N-乙酰半乳糖胺结合的小干扰 RNA(siRNA),旨在直接抑制肝细胞中 LPA 信使 RNA 的翻译,并有效降低血浆脂蛋白(a)的浓度。Olpasiran 能以剂量反应的方式降低转基因小鼠和金丝猴的脂蛋白(a)浓度,单次给药后 5-8 周,脂蛋白(a)浓度可从基线降低 80% 以上。在奥帕西兰的 1 期剂量递增试验(ClinicalTrials.gov:NCT03626662)中,主要结果是安全性和耐受性,次要结果是脂蛋白(a)浓度和奥帕西兰药代动力学参数的变化。参试者对单剂量奥帕西兰的耐受性良好,脂蛋白(a)浓度降低了71%-97%,服用9毫克或更高剂量后效果可持续数月。血清中奥帕西兰的浓度大致按剂量比例增加。总之,这些结果验证了使用肝细胞靶向 siRNA 有效降低血浆脂蛋白(a)浓度的方法。针对与冠状动脉疾病有关的脂蛋白(a)的小干扰 RNA 可在临床前模型和一期临床试验中持久降低脂蛋白(a)水平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
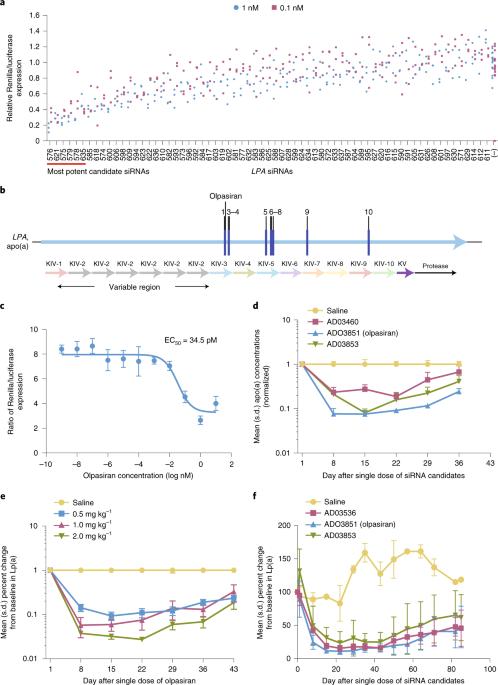
Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a)
Compelling evidence supports a causal role for lipoprotein(a) (Lp(a)) in cardiovascular disease. No pharmacotherapies directly targeting Lp(a) are currently available for clinical use. Here we report the discovery and development of olpasiran, a first-in-class, synthetic, double-stranded, N-acetylgalactosamine-conjugated small interfering RNA (siRNA) designed to directly inhibit LPA messenger RNA translation in hepatocytes and potently reduce plasma Lp(a) concentration. Olpasiran reduced Lp(a) concentrations in transgenic mice and cynomolgus monkeys in a dose-responsive manner, achieving up to over 80% reduction from baseline for 5–8 weeks after administration of a single dose. In a phase 1 dose-escalation trial of olpasiran (ClinicalTrials.gov: NCT03626662 ), the primary outcome was safety and tolerability, and the secondary outcomes were the change in Lp(a) concentrations and olpasiran pharmacokinetic parameters. Participants tolerated single doses of olpasiran well and experienced a 71–97% reduction in Lp(a) concentration with effects persisting for several months after administration of doses of 9 mg or higher. Serum concentrations of olpasiran increased approximately dose proportionally. Collectively, these results validate the approach of using hepatocyte-targeted siRNA to potently lower Lp(a) in individuals with elevated plasma Lp(a) concentration. A small-interfering RNA targeting lipoprotein(a), which is implicated in coronary artery disease, durably reduces lipoprotein(a) levels in preclinical models and in a phase 1 clinical trial.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


