单蛋白质弹性在机械生物学中的作用
IF 79.8
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 4
摘要
除了生化信号和遗传因素外,机械力正迅速成为人体生理的主要调节因素。然而,相比之下,人们对在细胞、组织或器官等广泛范围内调节力诱导功能的分子机制还不甚了解。随着单分子纳米力学技术的出现、发展和完善,我们已开始全面了解调节单个蛋白质弹性的各种物理化学原理。在此,我们回顾了目前在理解单个蛋白质的弹性如何调节机械感应和机械传导方面取得的主要进展。我们还讨论了这个多产且蓬勃发展的领域目前存在的局限性和未来面临的挑战。机械力可以调节单个蛋白质的构象和功能,这也是许多机械驱动的细胞过程的基础。本综述探讨了对已知在真核细胞机械感应和机械传导中发挥作用的蛋白质进行的单分子力谱实验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
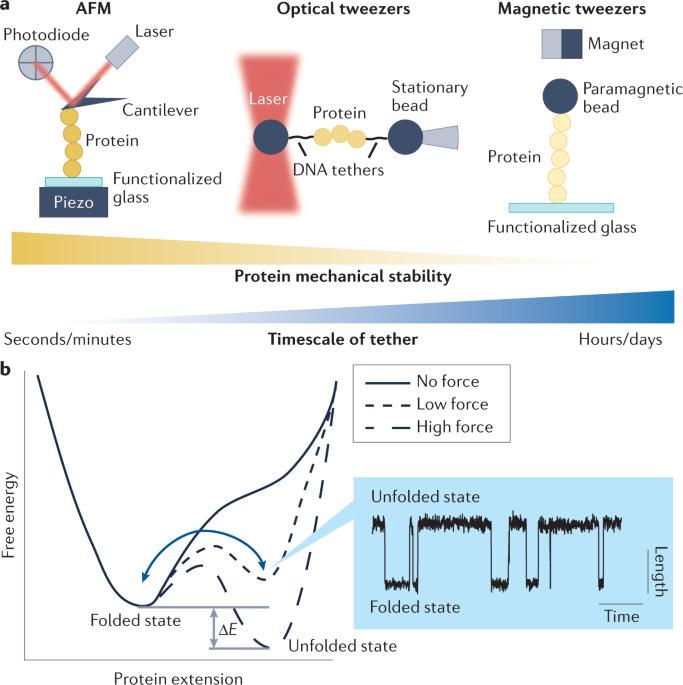
The role of single-protein elasticity in mechanobiology
In addition to biochemical signals and genetic considerations, mechanical forces are rapidly emerging as a master regulator of human physiology. However, the molecular mechanisms that regulate force-induced functionalities across a wide range of scales, encompassing the cell, tissue or organ levels, are not well understood in comparison. With the advent, development and refining of single-molecule nanomechanical techniques that enable the conformational dynamics of individual proteins under the effect of a calibrated force to be probed, we have begun to acquire a comprehensive knowledge of the diverse physicochemical principles that regulate the elasticity of single proteins. Here, we review the major advances underpinning our current understanding of how the elasticity of single proteins regulates mechanosensing and mechanotransduction. We discuss the present limitations and future challenges of this prolific and burgeoning field. Mechanical force modulates the conformation and function of individual proteins, and this underpins many mechanically driven cellular processes. This Review addresses single-molecule force spectroscopy experiments conducted on proteins with a known role in mechanosensing and mechanotransduction in eukaryotic cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Materials
Materials Science-Biomaterials
CiteScore
119.40
自引率
0.40%
发文量
107
期刊介绍:
Nature Reviews Materials is an online-only journal that is published weekly. It covers a wide range of scientific disciplines within materials science. The journal includes Reviews, Perspectives, and Comments.
Nature Reviews Materials focuses on various aspects of materials science, including the making, measuring, modelling, and manufacturing of materials. It examines the entire process of materials science, from laboratory discovery to the development of functional devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


