粪便微生物群移植联合抗PD-1免疫疗法治疗晚期黑色素瘤:一项I期试验。
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 8
摘要
粪便微生物群移植(FMT)是克服难治性黑色素瘤患者对免疫检查点抑制剂耐药性的一种潜在策略;然而,FMT在一线治疗环境中的作用尚未得到评估。我们在20名既往未经治疗的晚期黑色素瘤患者中进行了一项多中心I期试验,将健康供体FMT与PD-1抑制剂nivolumab或pembrolizumab相结合。主要的终点是安全。仅FMT未报告3级不良事件。5名患者(25%)在联合治疗中出现3级免疫相关不良事件。关键的次要终点是客观反应率、肠道微生物组组成的变化以及系统免疫和代谢组学分析。客观应答率为65%(20个中的13个),包括4个(20%)完全应答。纵向微生物组分析显示,所有患者都移植了来自各自捐赠者的菌株;然而,在应答者中,供体和患者微生物组之间的后天相似性只会随着时间的推移而增加。FMT后,应答者经历了免疫原性富集和有害细菌损失。Avatar小鼠模型证实了健康供体粪便在提高抗PD-1疗效方面的作用。我们的研究结果表明,来自健康捐赠者的FMT在一线环境中是安全的,需要与免疫检查点抑制剂联合进行进一步研究。ClinicalTrials.gov标识符NCT03772899。本文章由计算机程序翻译,如有差异,请以英文原文为准。
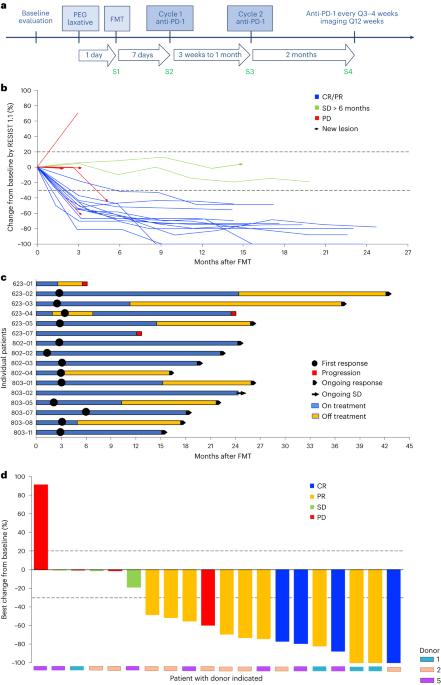
Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial
Fecal microbiota transplantation (FMT) represents a potential strategy to overcome resistance to immune checkpoint inhibitors in patients with refractory melanoma; however, the role of FMT in first-line treatment settings has not been evaluated. We conducted a multicenter phase I trial combining healthy donor FMT with the PD-1 inhibitors nivolumab or pembrolizumab in 20 previously untreated patients with advanced melanoma. The primary end point was safety. No grade 3 adverse events were reported from FMT alone. Five patients (25%) experienced grade 3 immune-related adverse events from combination therapy. Key secondary end points were objective response rate, changes in gut microbiome composition and systemic immune and metabolomics analyses. The objective response rate was 65% (13 of 20), including four (20%) complete responses. Longitudinal microbiome profiling revealed that all patients engrafted strains from their respective donors; however, the acquired similarity between donor and patient microbiomes only increased over time in responders. Responders experienced an enrichment of immunogenic and a loss of deleterious bacteria following FMT. Avatar mouse models confirmed the role of healthy donor feces in increasing anti-PD-1 efficacy. Our results show that FMT from healthy donors is safe in the first-line setting and warrants further investigation in combination with immune checkpoint inhibitors. ClinicalTrials.gov identifier NCT03772899 . In patients with advanced melanoma, fecal microbiota transplantation from healthy donors combined with the anti-PD-1 inhibitors nivolumab or pembrolizumab was well tolerated with an encouraging objective response rate of 65% in the first-line treatment setting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


