Rh(II)催化N-磺酰基-1,2,3-三唑与喹诺酮类和异喹诺酮类的脱氮反应。
IF 3.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们开发了一种从现成的N-磺酰基-1,2,3-三唑类和2-喹诺酮类或1-异喹诺酮类中获得生物相关的2-氨基喹啉和1-氨基异喹啉的有效方法。这种转化涉及这些衍生物在 原位生成Rh-氮杂乙烯基卡宾(Rh-AVC),然后进行重排。反应在操作简单的条件下顺利进行,并且发现该方案是可扩展的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
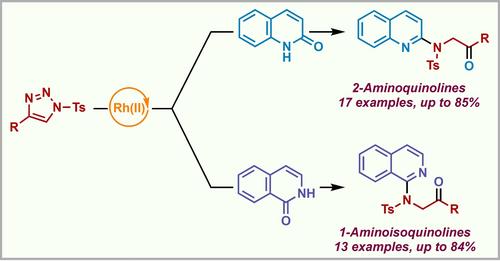
Rh(II)-Catalyzed Denitrogenative Reaction of N-Sulfonyl-1,2,3-triazoles with Quinolones and Isoquinolones
Herein, we developed an efficient approach to access biologically relevant 2-aminoquinolines and 1-aminoisoquinolines from readily available N-sulfonyl-1,2,3-triazoles and 2-quinolones or 1-isoquinolones. This transformation involves the selective O−H insertion of these derivatives onto the in situ generated Rh-azavinyl carbenes (Rh-AVC) followed by rearrangement. The reaction proceeds smoothly under operationally simple conditions and the protocol was found to be scalable.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry - An Asian Journal
化学-化学综合
CiteScore
7.00
自引率
2.40%
发文量
535
审稿时长
1.3 months
期刊介绍:
Chemistry—An Asian Journal is an international high-impact journal for chemistry in its broadest sense. The journal covers all aspects of chemistry from biochemistry through organic and inorganic chemistry to physical chemistry, including interdisciplinary topics.
Chemistry—An Asian Journal publishes Full Papers, Communications, and Focus Reviews.
A professional editorial team headed by Dr. Theresa Kueckmann and an Editorial Board (headed by Professor Susumu Kitagawa) ensure the highest quality of the peer-review process, the contents and the production of the journal.
Chemistry—An Asian Journal is published on behalf of the Asian Chemical Editorial Society (ACES), an association of numerous Asian chemical societies, and supported by the Gesellschaft Deutscher Chemiker (GDCh, German Chemical Society), ChemPubSoc Europe, and the Federation of Asian Chemical Societies (FACS).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


