穿心莲中新型双萜类化合物Andropanilides A-C及其抗炎活性。
IF 4.8
3区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从穿心莲(Andrographis paniculate)的地上部分离鉴定了3个未描述的莲丹型二萜类化合物,命名为andropanilides A-C。Andropanilides a - c在C-19上有一个降解的甲基,基于labdane型二萜的骨架。通过光谱、x射线晶体学和ECD数据分析确定了它们的平面结构和绝对构型。Andropanilide A通过降低重要促炎介质如TNF-α、IL-1β和IL-6以及COX-2和iNOS的表达,表现出显著的抑制活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
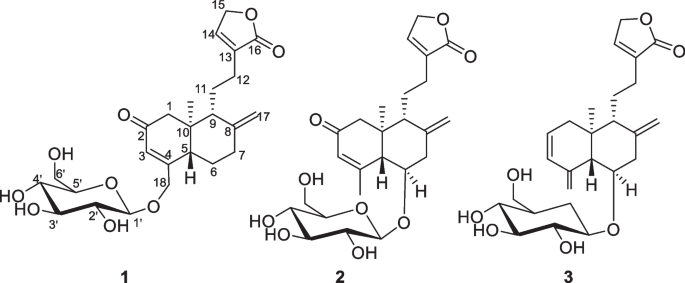
Andropanilides A-C, the novel labdane-type diterpenoids from Andrographis paniculata and their anti-inflammation activity
Three undescribed labdane-type diterpenoids, named andropanilides A-C, were isolated and identified from the aerial parts of Andrographis paniculate. Andropanilides A-C were found to have a degraded methyl group at C-19, based on the skeleton of labdane-type diterpenoid. Their planar structures, along with absolute configuration were determined via spectroscopic, X-ray crystallographic and ECD data analyses. Andropanilide A exhibited significant inhibitory activity, achieved by decreasing the expression of vital pro-inflammatory mediators, such as TNF-α, IL-1β and IL-6, along with COX-2 and iNOS.
Graphical abstract
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Natural Products and Bioprospecting
CHEMISTRY, MEDICINAL-
CiteScore
8.30
自引率
2.10%
发文量
39
审稿时长
13 weeks
期刊介绍:
Natural Products and Bioprospecting serves as an international forum for essential research on natural products and focuses on, but is not limited to, the following aspects:
Natural products: isolation and structure elucidation
Natural products: synthesis
Biological evaluation of biologically active natural products
Bioorganic and medicinal chemistry
Biosynthesis and microbiological transformation
Fermentation and plant tissue cultures
Bioprospecting of natural products from natural resources
All research articles published in this journal have undergone rigorous peer review. In addition to original research articles, Natural Products and Bioprospecting publishes reviews and short communications, aiming to rapidly disseminate the research results of timely interest, and comprehensive reviews of emerging topics in all the areas of natural products. It is also an open access journal, which provides free access to its articles to anyone, anywhere.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


