非酸性和非金属条件下3-硝基吲哚的区域选择性合成
IF 3.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了吲哚与硝酸四甲基铵在无酸、无金属条件下制备3-硝基吲哚的亲电取代反应。在本方案中,由硝酸四甲基铵和三氟乙酸酐在亚室温下进行复分解生成硝酸三氟乙酰基(CF3COONO2)。硝酸三氟乙酰基(CF3COONO2)是一种具有多种吲哚、芳香族和杂环芳香性的亲电硝化剂。同时,该策略可用于构建多种生物活性分子的骨架结构。有趣的是,3-硝基吲哚可以进一步衍生为吡咯[2,3-b]吲哚。本文章由计算机程序翻译,如有差异,请以英文原文为准。
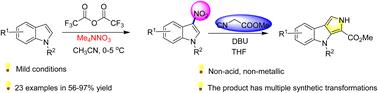
Regioselective synthesis of 3-nitroindoles under non-acidic and non-metallic conditions†
An electrophilic substitution reaction, without acid and metal, of indole with ammonium tetramethylnitrate for accessing 3-nitroindole has been developed. In this protocol, trifluoroacetyl nitrate (CF3COONO2) was produced by metathesis of ammonium tetramethyl nitrate and trifluoroacetic anhydride at sub-room temperature. Trifluoroacetyl nitrate (CF3COONO2) is an electrophilic nitrating agent for a variety of indoles, aromatic and heterocyclic aromaticity. Meanwhile, this strategy could be applied to construct the skeleton structure of many kinds of bioactive molecules. Interestingly, 3-nitroindole can be further derivatived as a pyrrolo[2,3-b]indole.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


