肿瘤诱导的T细胞沙漠化和排斥对免疫检查点疗法的耐药性:关键机制、预测和新的治疗机会。
IF 5.3
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫检查点疗法(ICT)可以重振抗肿瘤T细胞的效应功能,改善癌症患者的预后。抗肿瘤T细胞最初由抗原呈递细胞(APC)在其与肿瘤抗原的第一次接触(引发)期间形成。不幸的是,许多患者对ICT难以治疗,因为他们的肿瘤被认为是“冷”肿瘤——也就是说,他们不允许产生T细胞(所谓的“沙漠”肿瘤)或浸润现有的抗肿瘤T细胞(T细胞除外的肿瘤)。沙漠肿瘤通过利用源自其遗传不稳定性的抑制性肿瘤因子靶向APC来干扰抗原处理和T细胞的启动。相反,包括T细胞的肿瘤的特征是通过障碍物(如纤维化和肿瘤细胞诱导的免疫抑制)阻断有效的抗肿瘤T淋巴细胞浸润癌症肿块。这篇综述深入探讨了癌症细胞在ICT难治性肿瘤中诱导T细胞“荒漠化”和“排斥”的关键机制。填补我们对这些促肿瘤机制的认识空白将有助于研究人员开发新的免疫疗法,旨在通过APC和白细胞肿瘤运输更有效地启动来恢复T细胞的生成。这样的发展有望释放ICT对难治性患者的临床益处。本文章由计算机程序翻译,如有差异,请以英文原文为准。
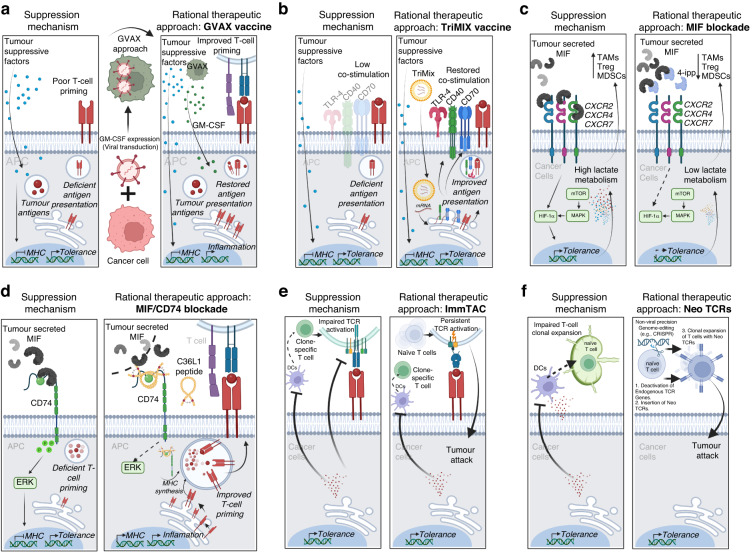
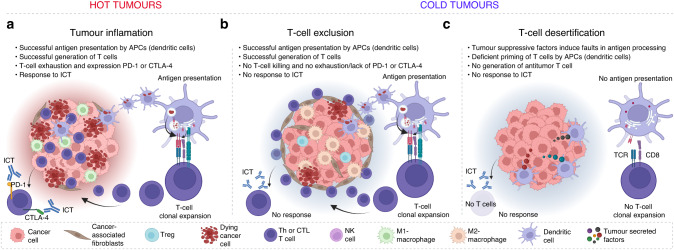
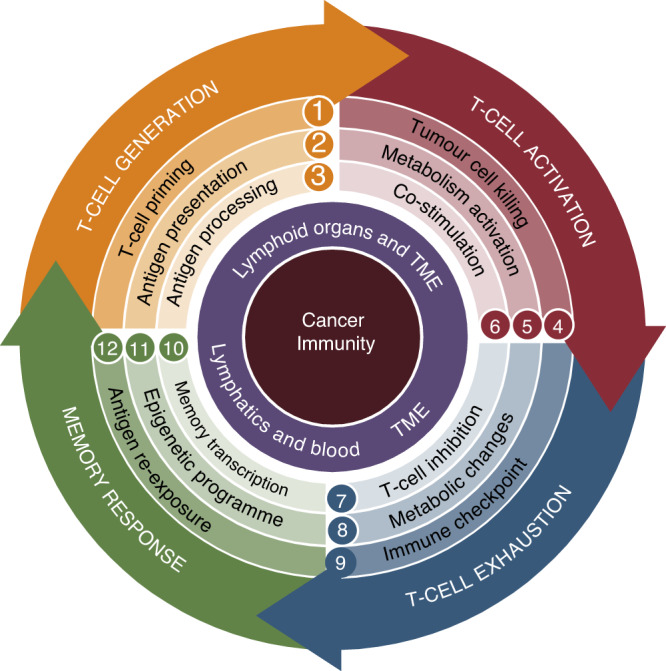
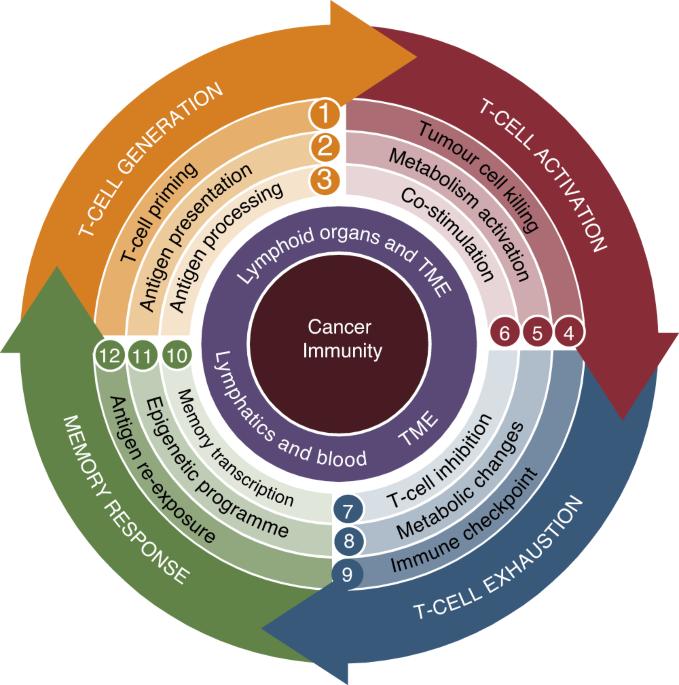
Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: key mechanisms, prognostication and new therapeutic opportunities
Immune checkpoint therapies (ICT) can reinvigorate the effector functions of anti-tumour T cells, improving cancer patient outcomes. Anti-tumour T cells are initially formed during their first contact (priming) with tumour antigens by antigen-presenting cells (APCs). Unfortunately, many patients are refractory to ICT because their tumours are considered to be ‘cold’ tumours—i.e., they do not allow the generation of T cells (so-called ‘desert’ tumours) or the infiltration of existing anti-tumour T cells (T-cell-excluded tumours). Desert tumours disturb antigen processing and priming of T cells by targeting APCs with suppressive tumour factors derived from their genetic instabilities. In contrast, T-cell-excluded tumours are characterised by blocking effective anti-tumour T lymphocytes infiltrating cancer masses by obstacles, such as fibrosis and tumour-cell-induced immunosuppression. This review delves into critical mechanisms by which cancer cells induce T-cell ‘desertification’ and ‘exclusion’ in ICT refractory tumours. Filling the gaps in our knowledge regarding these pro-tumoral mechanisms will aid researchers in developing novel class immunotherapies that aim at restoring T-cell generation with more efficient priming by APCs and leukocyte tumour trafficking. Such developments are expected to unleash the clinical benefit of ICT in refractory patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Nano Materials
Multiple-
CiteScore
8.30
自引率
3.40%
发文量
1601
期刊介绍:
ACS Applied Nano Materials is an interdisciplinary journal publishing original research covering all aspects of engineering, chemistry, physics and biology relevant to applications of nanomaterials. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important applications of nanomaterials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


