估计类黄酮氧化电位:机制和电荷相关的回归模型。
IF 1.6
4区 医学
Q3 PUBLIC, ENVIRONMENTAL & OCCUPATIONAL HEALTH
Arhiv Za Higijenu Rada I Toksikologiju-Archives of Industrial Hygiene and Toxicology
Pub Date : 2023-06-01
DOI:10.2478/aiht-2023-74-3721
引用次数: 0
摘要
在本文中,我测试了基于自旋居群的二次回归模型,用于估计类黄酮的氧化电位,类黄酮的阳离子和中性类黄酮之间的净原子电荷差异,类黄酮的自由基和阴离子之间的差异,以及类黄酮的自由基和中性类黄酮之间的差异(N = 35)。通过添加6种新的黄酮类化合物(5,6,7-三羟基黄酮,3,3',4',7-四羟基黄酮,3,7-二羟基黄酮,4',7-二羟基黄酮,4',5,7-三羟基异黄酮和6-羟基黄酮),我们创建了35种黄酮类化合物的校准集,并由同一实验人员在相同条件下测量了它们的氧化电位。最佳模型是基于净原子电荷差异的三个变量的平均值(r2 = 0.970, S.E. = 0.043),这与三种不同的电化学氧化机制,SET-PT, SPLET和HAT有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
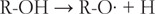
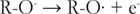
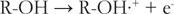
Estimating flavonoid oxidation potentials: mechanisms and charge-related regression models.
Abstract In this paper, I tested our quadratic regression models for the estimation of flavonoid oxidation potentials based on spin populations, the differences in the net atomic charges between a cation and a neutral flavonoid, between a radical and an anion of a flavonoid, and between a radical and a neutral flavonoid on a larger set of flavonoids (N = 35). By including six new flavonoids (5,6,7-trihydroxyflavone, 3,3’,4’,7-tetrahydroxyflavone, 3,7-dihydroxyflavone, 4’,7-dihydroxyflavone, 4’,5,7-trihydroxyisoflavone, and 6-hydroxyflavone), we created a respectable calibration set of 35 flavonoids with their oxidation potentials all measured at the same conditions by the same experimentalist. The best model was based on the mean values of the three variables using differences in the net atomic charges (R2 = 0.970, S.E. = 0.043), which are connected with the three different mechanisms of electrochemical oxidation, SET-PT, SPLET, and HAT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Arhiv Za Higijenu Rada I Toksikologiju-Archives of Industrial Hygiene and Toxicology
PUBLIC, ENVIRONMENTAL & OCCUPATIONAL HEALTH-TOXICOLOGY
CiteScore
3.50
自引率
4.80%
发文量
26
审稿时长
6-12 weeks
期刊介绍:
Archives of Industrial Hygiene and Toxicology (abbr. Arh Hig Rada Toksikol) is a peer-reviewed biomedical scientific quarterly that publishes contributions relevant to all aspects of environmental and occupational health and toxicology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


