Mizuho Kittaka, Tetsuya Yoshimoto, Marcus E Levitan, Rina Urata, Roy B Choi, Yayoi Teno, Yixia Xie, Yukiko Kitase, Matthew Prideaux, Sarah L Dallas, Alexander G Robling, Yasuyoshi Ueki
下载PDF
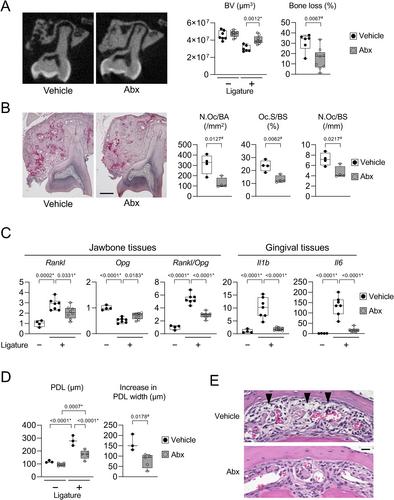
{"title":"骨细胞RANKL在小鼠连字诱导的牙周炎中驱动骨吸收","authors":"Mizuho Kittaka, Tetsuya Yoshimoto, Marcus E Levitan, Rina Urata, Roy B Choi, Yayoi Teno, Yixia Xie, Yukiko Kitase, Matthew Prideaux, Sarah L Dallas, Alexander G Robling, Yasuyoshi Ueki","doi":"10.1002/jbmr.4897","DOIUrl":null,"url":null,"abstract":"<p>Mouse ligature-induced periodontitis (LIP) has been used to study bone loss in periodontitis. However, the role of osteocytes in LIP remains unclear. Furthermore, there is no consensus on the choice of alveolar bone parameters and time points to evaluate LIP. Here, we investigated the dynamics of changes in osteoclastogenesis and bone volume (BV) loss in LIP over 14 days. Time-course analysis revealed that osteoclast induction peaked on days 3 and 5, followed by the peak of BV loss on day 7. Notably, BV was restored by day 14. The bone formation phase after the bone resorption phase was suggested to be responsible for the recovery of bone loss. Electron microscopy identified bacteria in the osteocyte lacunar space beyond the periodontal ligament (PDL) tissue. We investigated how osteocytes affect bone resorption of LIP and found that mice lacking receptor activator of NF-κB ligand (RANKL), predominantly in osteocytes, protected against bone loss in LIP, whereas recombination activating 1 (RAG1)-deficient mice failed to resist it. These results indicate that T/B cells are dispensable for osteoclast induction in LIP and that RANKL from osteocytes and mature osteoblasts regulates bone resorption by LIP. Remarkably, mice lacking the myeloid differentiation primary response gene 88 (MYD88) did not show protection against LIP-induced bone loss. Instead, osteocytic cells expressed nucleotide-binding oligomerization domain containing 1 (NOD1), and primary osteocytes induced significantly higher <i>Rankl</i> than primary osteoblasts when stimulated with a NOD1 agonist. Taken together, LIP induced both bone resorption and bone formation in a stage-dependent manner, suggesting that the selection of time points is critical for quantifying bone loss in mouse LIP. Pathogenetically, the current study suggests that bacterial activation of osteocytes via NOD1 is involved in the mechanism of osteoclastogenesis in LIP. The NOD1-RANKL axis in osteocytes may be a therapeutic target for bone resorption in periodontitis. © 2023 The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>","PeriodicalId":185,"journal":{"name":"Journal of Bone and Mineral Research","volume":"38 10","pages":"1521-1540"},"PeriodicalIF":5.1000,"publicationDate":"2023-08-08","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4897","citationCount":"0","resultStr":"{\"title\":\"Osteocyte RANKL Drives Bone Resorption in Mouse Ligature-Induced Periodontitis\",\"authors\":\"Mizuho Kittaka, Tetsuya Yoshimoto, Marcus E Levitan, Rina Urata, Roy B Choi, Yayoi Teno, Yixia Xie, Yukiko Kitase, Matthew Prideaux, Sarah L Dallas, Alexander G Robling, Yasuyoshi Ueki\",\"doi\":\"10.1002/jbmr.4897\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Mouse ligature-induced periodontitis (LIP) has been used to study bone loss in periodontitis. However, the role of osteocytes in LIP remains unclear. Furthermore, there is no consensus on the choice of alveolar bone parameters and time points to evaluate LIP. Here, we investigated the dynamics of changes in osteoclastogenesis and bone volume (BV) loss in LIP over 14 days. Time-course analysis revealed that osteoclast induction peaked on days 3 and 5, followed by the peak of BV loss on day 7. Notably, BV was restored by day 14. The bone formation phase after the bone resorption phase was suggested to be responsible for the recovery of bone loss. Electron microscopy identified bacteria in the osteocyte lacunar space beyond the periodontal ligament (PDL) tissue. We investigated how osteocytes affect bone resorption of LIP and found that mice lacking receptor activator of NF-κB ligand (RANKL), predominantly in osteocytes, protected against bone loss in LIP, whereas recombination activating 1 (RAG1)-deficient mice failed to resist it. These results indicate that T/B cells are dispensable for osteoclast induction in LIP and that RANKL from osteocytes and mature osteoblasts regulates bone resorption by LIP. Remarkably, mice lacking the myeloid differentiation primary response gene 88 (MYD88) did not show protection against LIP-induced bone loss. Instead, osteocytic cells expressed nucleotide-binding oligomerization domain containing 1 (NOD1), and primary osteocytes induced significantly higher <i>Rankl</i> than primary osteoblasts when stimulated with a NOD1 agonist. Taken together, LIP induced both bone resorption and bone formation in a stage-dependent manner, suggesting that the selection of time points is critical for quantifying bone loss in mouse LIP. Pathogenetically, the current study suggests that bacterial activation of osteocytes via NOD1 is involved in the mechanism of osteoclastogenesis in LIP. The NOD1-RANKL axis in osteocytes may be a therapeutic target for bone resorption in periodontitis. © 2023 The Authors. <i>Journal of Bone and Mineral Research</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).</p>\",\"PeriodicalId\":185,\"journal\":{\"name\":\"Journal of Bone and Mineral Research\",\"volume\":\"38 10\",\"pages\":\"1521-1540\"},\"PeriodicalIF\":5.1000,\"publicationDate\":\"2023-08-08\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/jbmr.4897\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Journal of Bone and Mineral Research\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4897\",\"RegionNum\":1,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ENDOCRINOLOGY & METABOLISM\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of Bone and Mineral Research","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbmr.4897","RegionNum":1,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


