癌症抗雌激素治疗的耐药性。
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 2
摘要
激素受体雌激素受体-α(ER)协调生理性乳腺发育、乳腺癌发生和乳腺肿瘤发展为致命的、难治的系统性疾病。选择性拮抗ER信号是肿瘤学中最成功的治疗方法之一,作为癌症的预防措施和癌症的治疗策略,使患者受益。然而,抗雌激素治疗的耐药性是一个主要的临床挑战。在过去的十年里,我们已经了解了乳腺癌是如何在抗雌激素治疗的压力下发展的。编码ER(ESR1)基因的雌激素依赖性突变最能说明这一点,这种突变在原发性乳腺癌症中几乎不存在,但在抗雌激素治疗的转移性疾病中高度流行(20-40%)。这些和其他发现强调了ER+乳腺癌症的“进化性”,以及理解这种进化发生的分子过程的必要性。最近开发并批准了下一代ER拮抗剂来靶向ESR1型癌症,这突出了这种可进化性的临床重要性,并为ER+乳腺癌的治疗树立了新的范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
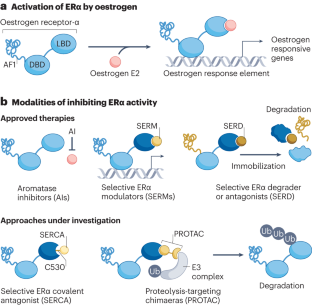
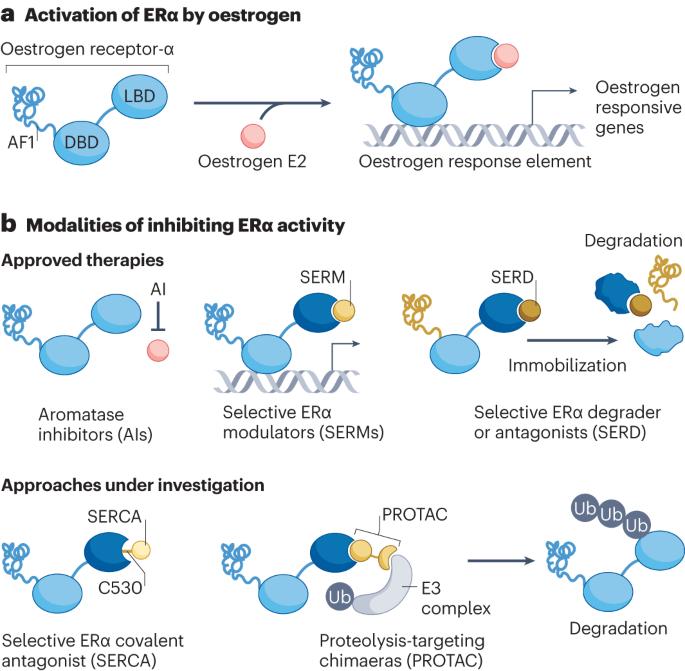
Therapeutic resistance to anti-oestrogen therapy in breast cancer
The hormone receptor oestrogen receptor-α (ER) orchestrates physiological mammary gland development, breast carcinogenesis and the progression of breast tumours into lethal, treatment-refractory systemic disease. Selective antagonism of ER signalling has been one of the most successful therapeutic approaches in oncology, benefiting patients as both a cancer preventative measure and a cancer treatment strategy. However, resistance to anti-oestrogen therapy is a major clinical challenge. Over the past decade, we have gained an understanding of how breast cancers evolve under the pressure of anti-oestrogen therapy. This is best depicted by the case of oestrogen-independent mutations in the gene encoding ER (ESR1), which are virtually absent in primary breast cancer but highly prevalent (20–40%) in anti-oestrogen-treated metastatic disease. These and other findings highlight the ‘evolvability’ of ER+ breast cancer and the need to understand molecular processes by which this evolution occurs. Recent development and approval of next-generation ER antagonists to target ESR1-mutant breast cancer underscores the clinical importance of this evolvability and sets a new paradigm for the treatment of ER+ breast cancers. Although selective antagonism of oestrogen receptor signalling in breast cancer has been one of the most successful therapeutic approaches in oncology, resistance is a major clinical challenge. In this Review, Will et al. explore mechanisms of oestrogen-receptor-α-targeted therapeutic resistance and strategies to overcome it.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


