LRG1 通过调节内皮 TGF-β 信号促进血管生成
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 347
摘要
血管新生异常是癌症、失明和动脉粥样硬化等疾病的诱因,也是不适当的血管新生信号的结果。虽然已经发现了许多致病性血管生成的调节因子,但我们对这一过程的了解还不全面。在这里,我们探索了从表现出血管病理学的视网膜疾病小鼠模型中分离出来的视网膜微血管的转录组,并发现了一个上调基因--富亮氨酸α-2-糖蛋白 1(Lrg1),其功能之前尚不清楚。我们发现,在转化生长因子-β1(TGF-β1)存在的情况下,LRG1 对内皮细胞具有有丝分裂作用,并促进血管生成。缺乏 LRG1 的小鼠会出现轻微的视网膜血管表型,但病理性眼部血管生成会显著减少。LRG1 直接与 TGF-β 辅助受体 endoglin 结合,在 TGF-β1 存在的情况下,促进血管生成的 Smad1/5/8 信号通路。LRG1 抗体阻断抑制了这一转换,并减弱了血管生成。这些研究揭示了一种新的血管生成调节因子,它通过调节 TGF-β 信号传导而产生作用。研究发现,LRG1 是一种新的 TGF-β 信号调节因子,它通过 TβRII-ALK1-ENG-Smad1/5/8 信号通路促进血管生成;在视网膜损伤的小鼠模型中,抗体介导的 LRG1 抑制可减少致病性新生血管生成。血管生成缺陷是许多疾病的共同特征,包括老年性黄斑变性、动脉粥样硬化、类风湿性关节炎和癌症。在这里,约翰-格林伍德及其同事发现了一种以前未知功能的新型血管生成糖蛋白--亮氨酸-富α-2-糖蛋白1(LRG1),它通过改变TGF-β信号发挥其作用。LRG1在患有增殖性糖尿病视网膜病变的人的玻璃体样本中上调,它通过与受体内皮素结合并促进促血管生成的TGF-β信号传导,从而激活血管生成开关。抗体介导的 LRG1 抑制剂可减少小鼠视网膜损伤模型中的致病性新生血管,这表明 LRG1 可能是控制眼部疾病病理性血管生成的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
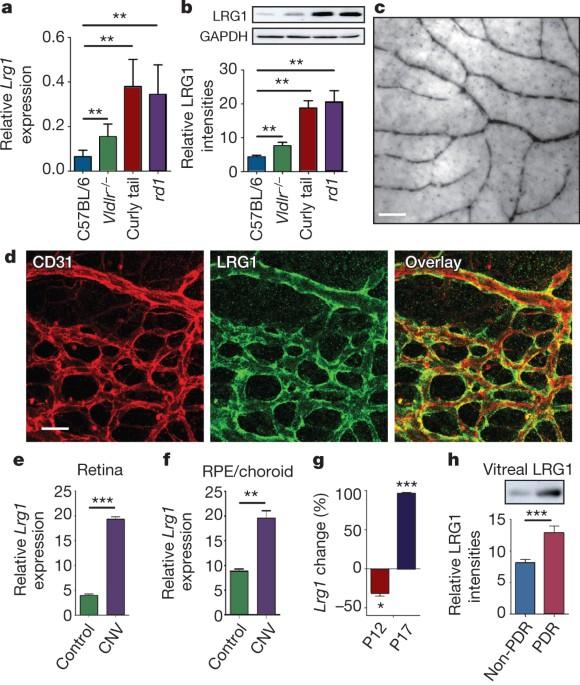
LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling
Aberrant neovascularization contributes to diseases such as cancer, blindness and atherosclerosis, and is the consequence of inappropriate angiogenic signalling. Although many regulators of pathogenic angiogenesis have been identified, our understanding of this process is incomplete. Here we explore the transcriptome of retinal microvessels isolated from mouse models of retinal disease that exhibit vascular pathology, and uncover an upregulated gene, leucine-rich alpha-2-glycoprotein 1 (Lrg1), of previously unknown function. We show that in the presence of transforming growth factor-β1 (TGF-β1), LRG1 is mitogenic to endothelial cells and promotes angiogenesis. Mice lacking Lrg1 develop a mild retinal vascular phenotype but exhibit a significant reduction in pathological ocular angiogenesis. LRG1 binds directly to the TGF-β accessory receptor endoglin, which, in the presence of TGF-β1, results in promotion of the pro-angiogenic Smad1/5/8 signalling pathway. LRG1 antibody blockade inhibits this switch and attenuates angiogenesis. These studies reveal a new regulator of angiogenesis that mediates its effect by modulating TGF-β signalling. LRG1 is identified as a new regulator of TGF-β signalling that promotes angiogenesis via a TβRII–ALK1–ENG–Smad1/5/8 signalling pathway; antibody-mediated inhibition of LRG1 reduces pathogenic neovascularization in a mouse model of retinal injury. Defective angiogenesis is a common feature in many diseases including age-related macular degeneration, atherosclerosis, rheumatoid arthritis and cancer. Here John Greenwood and colleagues identify a novel angiogenic glycoprotein of previously unknown function — leucine-rich-alpha-2-glycoprotein 1 (LRG1) — that exerts its effect through modifying TGF-β signalling. LRG1, upregulated in vitreous samples from humans with proliferative diabetic retinopathy, activates an angiogenic switch by binding to the receptor endoglin and promoting pro-angiogenic TGF-β signalling. Antibody-mediated inhibition of LRG1 reduces pathogenic neovascularization in a mouse model of retinal injury, which suggests that LRG1 is a possible therapeutic target for controlling pathological angiogenesis in ocular disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


