非侵入性激活瘤内基因编辑,改进实体瘤的收养 T 细胞疗法
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 6
摘要
针对实体瘤的适应性 T 细胞疗法受到肿瘤细胞凋亡抵抗机制以及细胞外免疫抑制性肿瘤微环境的限制。在这里,我们报告了一种对温度敏感的基因组编辑纳米装置,它可以通过外部触发器递送 Cas9 编辑器,用于编辑肿瘤细胞的基因组,从而降低细胞凋亡的抵抗力,并通过温和的加热触发器调节肿瘤微环境。在局部或全身给药 Cas9 后,通过非侵入性的近红外(NIR)光或聚焦超声(FUS)诱导轻度加热来激活 Cas9,从而同时启动肿瘤细胞中 HSP70 (HSPA1A) 和 BAG3 的基因组编辑。这就破坏了肿瘤细胞抵抗收养 T 细胞的凋亡机制。同时,近红外或 FUS 诱导的温和热效应通过破坏物理屏障和免疫抑制重塑了细胞外肿瘤微环境。这有利于被收养 T 细胞的浸润,并增强其治疗活性。在不同的小鼠肿瘤模型(包括基于人源化患者异种移植的肿瘤模型)中,模拟一系列临床适应症的轻度热Cas9递送得到了证实。因此,Cas9 的非侵入性热传递可显著提高肿瘤浸润淋巴细胞和嵌合抗原受体 T 的治疗效果,并显示出临床应用的潜力。癌症对细胞凋亡的抵抗会阻碍基于 T 细胞的疗法。在这里,作者开发了一种对温度敏感的系统,用于控制针对抗性机制 HSP70 和 BAG3 的 Cas9 基因编辑序列的递送,该系统具有温和的热效应,可增加 T 细胞的递送和治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
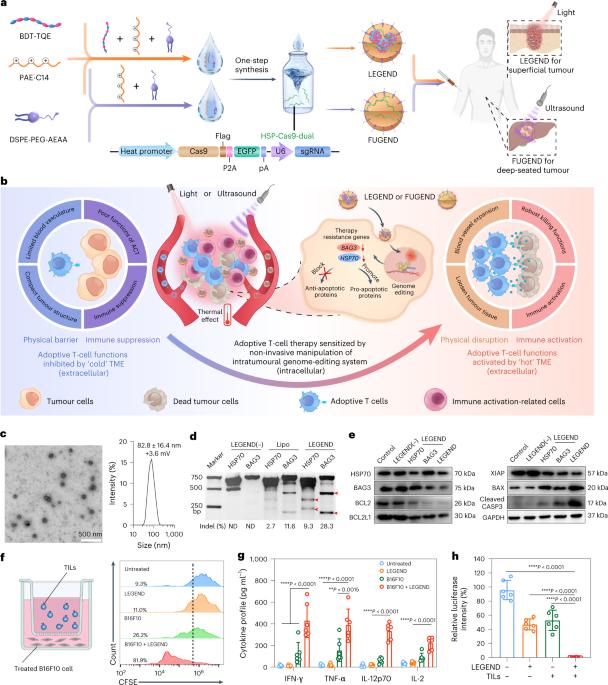
Non-invasive activation of intratumoural gene editing for improved adoptive T-cell therapy in solid tumours
Adoptive T-cell therapy against solid tumours is limited by the apoptosis resistance mechanisms of tumour cells and by the extracellular, immunosuppressive tumour microenvironment. Here we report a temperature-sensitive genome-editing nanodevice that can deliver a Cas9 editor with an external trigger which can be used to edit the genome of tumour cells to reduce resistance to apoptosis and modulate the tumour microenvironment via a mild heating trigger. After local or systemic delivery of Cas9, mild heating is induced by non-invasive near-infrared (NIR) light or focused ultrasound (FUS) to activate Cas9, which initiates simultaneous genome editing of HSP70 (HSPA1A) and BAG3 in tumour cells. This disrupts the apoptotic resistance machinery of the tumour cells against adoptive T cells. At the same time, an NIR- or FUS-induced mild thermal effect reshapes the extracellular tumour microenvironment by disrupting the physical barriers and immune suppression. This facilitates the infiltration of adoptive T cells and enhances their therapeutic activity. Mild thermal Cas9 delivery is demonstrated in different murine tumour models which mimic a range of clinical indications, including a tumour model based on humanized patient-derived xenografts. As a result, the non-invasive thermal delivery of Cas9 significantly enhances the therapeutic efficacies of tumour-infiltrating lymphocytes and chimeric antigen receptor T and shows potential for clinical application. Cancer resistance to apoptosis can hinder T-cell-based therapies. Here, the authors develop a temperature-sensitive system for the controlled delivery of a Cas9 gene-editing sequence targeting resistance mechanisms HSP70 and BAG3, which with a mild thermal effect increases T-cell delivery and therapeutic outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


