Remote neuronal activity drives glioma progression through SEMA4F
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 6
Abstract
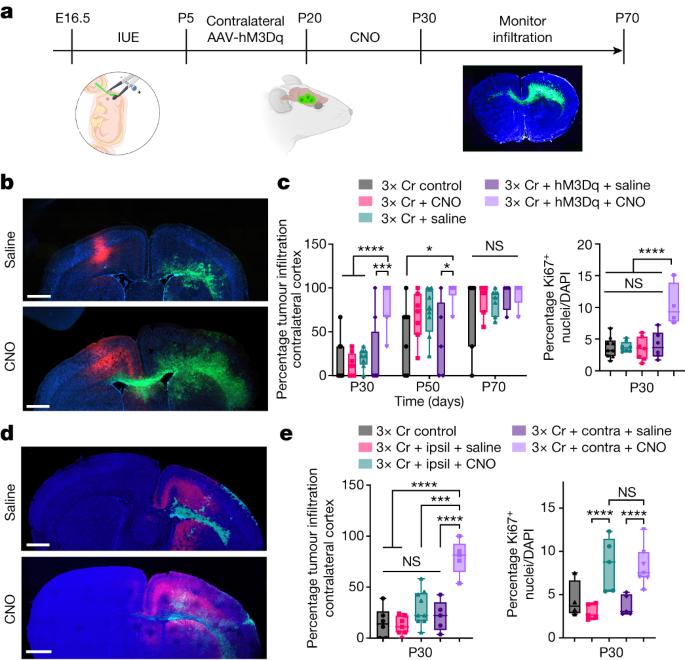
The tumour microenvironment plays an essential role in malignancy, and neurons have emerged as a key component of the tumour microenvironment that promotes tumourigenesis across a host of cancers1,2. Recent studies on glioblastoma (GBM) highlight bidirectional signalling between tumours and neurons that propagates a vicious cycle of proliferation, synaptic integration and brain hyperactivity3–8; however, the identity of neuronal subtypes and tumour subpopulations driving this phenomenon is incompletely understood. Here we show that callosal projection neurons located in the hemisphere contralateral to primary GBM tumours promote progression and widespread infiltration. Using this platform to examine GBM infiltration, we identified an activity-dependent infiltrating population present at the leading edge of mouse and human tumours that is enriched for axon guidance genes. High-throughput, in vivo screening of these genes identified SEMA4F as a key regulator of tumourigenesis and activity-dependent progression. Furthermore, SEMA4F promotes the activity-dependent infiltrating population and propagates bidirectional signalling with neurons by remodelling tumour-adjacent synapses towards brain network hyperactivity. Collectively our studies demonstrate that subsets of neurons in locations remote to primary GBM promote malignant progression, and also show new mechanisms of glioma progression that are regulated by neuronal activity. Callosal projection neurons located in the hemisphere contralateral to primary glioblastoma promote progression and widespread infiltration, and screening of axon guidance genes identified SEMA4F as a key regulator of tumourigenesis and activity-dependent progression.
远程神经元活动通过SEMA4F驱动神经胶质瘤进展。
肿瘤微环境在恶性肿瘤中起着重要作用,神经元已成为肿瘤微环境的关键组成部分,促进多种癌症的肿瘤发生1,2。最近对胶质母细胞瘤(GBM)的研究强调,肿瘤和神经元之间的双向信号传导会传播增殖、突触整合和大脑过度活跃的恶性循环3-8;然而,驱动这种现象的神经元亚型和肿瘤亚群的身份尚不完全清楚。在这里,我们发现位于原发性GBM肿瘤对侧半球的胼胝体投射神经元促进进展和广泛浸润。使用该平台检测GBM浸润,我们确定了一个存在于小鼠和人类肿瘤前沿的活性依赖性浸润群体,该群体富含轴突引导基因。这些基因的高通量体内筛选确定SEMA4F是肿瘤发生和活性依赖性进展的关键调节因子。此外,SEMA4F促进活性依赖性浸润群体,并通过重塑肿瘤附近的突触向脑网络过度活跃传播与神经元的双向信号。总之,我们的研究表明,远离原发性GBM的神经元亚群促进了恶性进展,也显示了受神经元活动调节的神经胶质瘤进展的新机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


