AAV1.NT-3 gene therapy in the SOD1KO mouse model of accelerated sarcopenia
Abstract
Background
Sarcopenia, an age-related loss of muscle mass, is a critical factor that affects the health of the older adults. The SOD1KO mouse is deficient of Cu/Zn superoxide dismutase, used as an accelerated aging model. We previously showed that NT-3 improves muscle fibre size by activating the mTOR pathway, suggesting a potential for attenuating age-related muscle loss. This study assessed the therapeutic efficacy of AAV1.NT-3 in this accelerated aging model.
Methods
Twelve 6 months old SOD1KO mice were injected intramuscularly with a 1 × 1011 vg dose of AAV1.tMCK.NT-3, and 13 age-matched SOD1KO mice were used as controls. The treatment effect was evaluated using treadmill, rotarod and gait analyses as well as histological studies assessing changes in muscle fibre, and fibre type switch, in tibialis anterior, gastrocnemius, and triceps muscles, and myelin thickness by calculating G ratio in sciatic and tibial nerves. Molecular studies involved qPCR experiments to analyse the expression levels of mitochondrial and glycolysis markers and western blot experiments to assess the activity of mTORC1 pathway.
Results
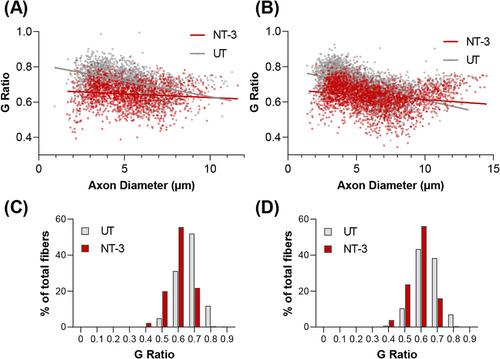
Treatment resulted in a 36% (154.9 vs. 114.1; P < 0.0001) and 76% increase (154.3 vs. 87.6; P < 0.0001) in meters ran, with treadmill test at 3 and 6 months post gene delivery. In addition, the treated cohort stayed on rotarod 30% (52.7 s vs. 40.4 s; P = 0.0095) and 54% (50.4 s vs. 32.7 s; P = 0.0007) longer, compared with untreated counterparts at 3 and 6 months post injection. Gait analysis, performed at endpoint, showed that stride width was normalized to wild type levels (29.3 mm) by an 11% decrease, compared with untreated cohort (28.6 mm vs. 32.1 mm; P = 0.0014). Compared with wild-type, SOD1KO mice showed 9.4% and 11.4% fibre size decrease in tibialis anterior and gastrocnemius muscles, respectively, which were normalized to wild type levels with treatment. Fibre diameter increase was observed prominently in FTG fibre type. G ratio analysis revealed hypomyelination in the tibial (0.721) and sciatic (0.676) nerves of SOD1KO model, which was reversed in the NT-3 cohort (0.646 and 0.634, respectively). Fibre size increase correlated with the increase in the p-S6 and p-4E-BP1 levels, and in the glycolysis markers in tibialis anterior. Alterations observed in the mitochondrial markers were not rescued with treatment. Overall, response to NT-3 was subdued in gastrocnemius muscle.
Conclusions
This study shows that AAV1.NT-3 gene therapy protected SOD1KO mouse from accelerated aging effects functionally and histologically. We further confirmed that NT-3 has potential to activate the mTOR and glycolytic pathways in muscle.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


