A retrospective analysis of immune checkpoint inhibitors in patients with preexisting organ dysfunction
Abstract
Background
There are limited to no data regarding the use of immune checkpoint inhibitors (ICIs) in patients who have preexisting organ dysfunction because these patients are frequently excluded from clinical trials. The authors’ objective was to evaluate the effects of ICIs in patients with chronic kidney disease (CKD), cirrhosis, chronic obstructive pulmonary disease (COPD), and congestive heart failure (CHF).
Methods
Data were obtained retrospectively for patients older than 18 years with solid organ malignancies who received at least one dose of an ICI between January 1, 2015, and January 1, 2021, and had either CKD (n = 90), cirrhosis (n = 20), COPD (n = 142), or CHF (n = 82) before ICI initiation at the authors’ institution. Descriptive statistics were used to summarize patient characteristics, treatment characteristics, immune-related adverse events (IrAEs), and outcomes. An independent samples t-test or the Wilcoxon rank-sum test was used to assess differences in continuous variables; the χ2 test or the Fisher exact test was used to assess differences in categorical variables between patients with and without IrAEs. Progression-free survival (PFS) was assessed using Kaplan–Meier curves, and the log-rank test was used to assess differences in PFS.
Results
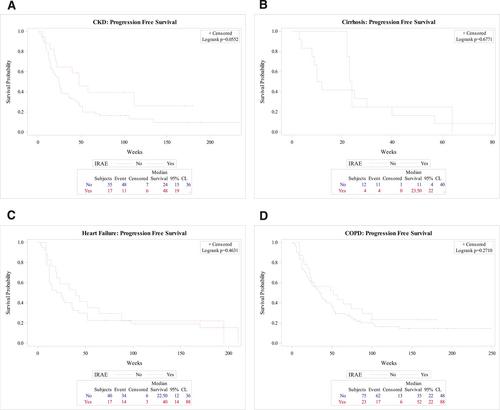
In all four cohorts, there were no statistically significant differences in patient characteristics, treatment characteristics, or outcomes, such as the number of hospitalizations and PFS, among those who experienced IrAEs compared with those who did not. In the CKD cohort, patients with IrAEs were significantly less likely to die than those without IrAEs (52% vs. 81% [p = .009] for all patients; 53% vs. 83% [p = .008] for patients with stage II/III disease who received no definitive local treatment and patients with stage IV disease); this difference was not observed in the cirrhosis, COPD, or CHF cohorts. There was no statistically significant difference in the number of heart failure and COPD exacerbations during the receipt of ICIs in the CHF and COPD cohorts, respectively. The incidence and time to onset of IrAEs in this study appeared to be similar to those reported previously in clinical trials that excluded patients with significant comorbidities.
Conclusions
The current results demonstrate that ICIs are well tolerated by patients who have preexisting organ dysfunction.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


