Identification and characterization of inulinases by bioinformatics analysis of bacterial glycoside hydrolases family 32 (GH32)
Abstract
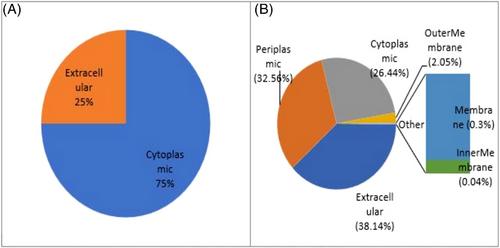
The glycoside hydrolase family contains enzymes that break the glycosidic bonds of carbohydrates by hydrolysis. Inulinase is one of the most important industrial enzymes in the family of Glycoside Hydrolases 32 (GH32). In this study, to identify and classify bacterial inulinases initially, 16,002 protein sequences belonging to the GH32 family were obtained using various databases. The inulin-effective enzymes (endoinulinase and exoinulinase) were identified. Eight endoinulinases (EC 3.2.1.7) and 4318 exoinulinases (EC 3.2.1.80) were found. Then, the localization of endoinulinase and exoinulinase enzymes in the cell was predicted. Among them, two extracellular endoinulinases and 1232 extracellular exoinulinases were found. The biochemical properties of 363 enzymes of the genus Arthrobacter, Bacillus, and Streptomyces (most abundant) showed that exoinulinases have an acid isoelectric point up to the neutral range due to their amino acid length. That is, the smaller the protein (336 aa), the more acidic the pI (4.39), and the larger the protein (1207 aa), the pI is in the neutral range (8.84). Also, a negative gravitational index indicates the hydrophilicity of exoinulinases. Finally, considering the biochemical properties affecting protein stability and post-translational changes studies, one enzyme for endoinulinase and 40 enzymes with desirable characteristics were selected to identify their enzyme production sources. To screen and isolate enzyme-containing strains, now with the expansion of databases and the development of bioinformatics tools, it is possible to classify, review and analyze a lot of data related to different enzyme-producing strains. Although, in laboratory studies, a maximum of 20 to 30 strains can be examined. Therefore, when more strains are examined, finally, strains with more stable and efficient enzymes were selected and introduced for laboratory activities. The findings of this study can help researchers to select the appropriate gene source from introduced strains for cloning and expression heterologous inulinase, or to extract native inulinase from introduced strains.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


