Self-maintaining macrophages within the kidney contribute to salt and water balance by modulating kidney sympathetic nerve activity
Abstract
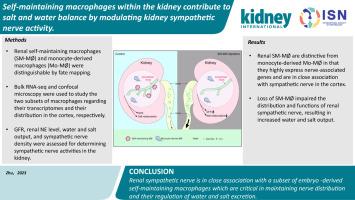
The kidney is critical in controlling salt and water balance, with the interstitium involved with a variety of components including immune cells in steady state. However, the roles of resident immune cells in kidney physiology are largely unknown. To help unravel some of these unknowns, we employed cell fate mapping, and identified a population of embryo-derived self-maintaining macrophages (SM-MØ) that were independent of the bone marrow in adult mouse kidneys. This kidney-specific SM-MØ population was distinctive from the kidney monocyte-derived macrophages in transcriptome and in their distribution. Specifically, the SM-MØ highly expressed nerve-associated genes; high-resolution confocal microscopy revealed that the SM-MØ in the cortex were in close association with sympathetic nerves and there was a dynamical interaction between macrophages and sympathetic nerves when live kidney sections were monitored. Kidney-specific depletion of the SM-MØ resulted in reduced sympathetic distribution and tone, leading to reduced renin secretion, increased glomerular filtration rate and solute diuresis, which caused salt decompensation and significant weight loss under a low-salt diet challenge. Supplementation of L-3,4-dihydroxyphenylserine which is converted to norepinephrine in vivo rescued the phenotype of SM-MØ-depleted mice. Thus, our findings provide insights in kidney macrophage heterogeneity and address a non-canonical role of macrophages in kidney physiology. In contrast to the well-appreciated way of central regulation, local regulation of sympathetic nerve distribution and activities in the kidney was uncovered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


