Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 2
Abstract
Intravenously administered cyclic dinucleotides and other STING agonists are hampered by low cellular uptake and poor circulatory half-life. Here we report the covalent conjugation of cyclic dinucleotides to poly(β-amino ester) nanoparticles through a cathepsin-sensitive linker. This is shown to increase stability and loading, thereby expanding the therapeutic window in multiple syngeneic tumour models, enabling the study of how the long-term fate of the nanoparticles affects the immune response. In a melanoma mouse model, primary tumour clearance depends on the STING signalling by host cells—rather than cancer cells—and immune memory depends on the spleen. The cancer cells act as a depot for the nanoparticles, releasing them over time to activate nearby immune cells to control tumour growth. Collectively, this work highlights the importance of nanoparticle structure and nano-biointeractions in controlling immunotherapy efficacy. STING agonists are often limited by low circulation time and cellular uptake. The conjugation of STING agonists with polymer nanoparticles is shown to enhance stability, circulation time and cellular uptake, increasing the immunotherapeutic activity.
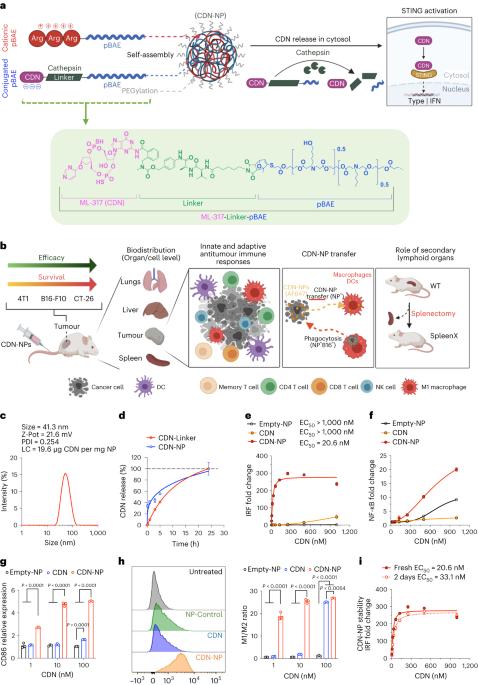
STING激动剂与纳米聚合物偶联后抗肿瘤效能的研究。
静脉注射环二核苷酸和其他STING激动剂受到细胞摄取低和循环半衰期差的阻碍。在这里,我们报道了环二核苷酸通过组织蛋白酶敏感连接物与聚(β-氨基酯)纳米颗粒的共价偶联。研究表明,这增加了稳定性和负荷,从而扩大了多同基因肿瘤模型的治疗窗口,使研究纳米颗粒的长期命运如何影响免疫反应成为可能。在黑色素瘤小鼠模型中,原发性肿瘤清除依赖于宿主细胞的STING信号,而不是癌细胞,免疫记忆依赖于脾脏。癌细胞充当了纳米颗粒的仓库,随着时间的推移释放它们来激活附近的免疫细胞来控制肿瘤的生长。总的来说,这项工作强调了纳米颗粒结构和纳米生物相互作用在控制免疫治疗疗效中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


