Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 4
Abstract
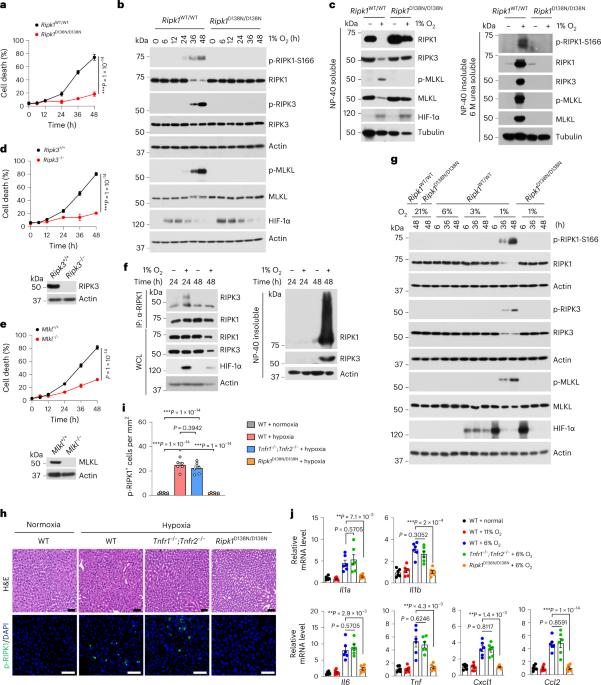
The prolyl hydroxylation of hypoxia-inducible factor 1α (HIF-1α) mediated by the EGLN–pVHL pathway represents a classic signalling mechanism that mediates cellular adaptation under hypoxia. Here we identify RIPK1, a known regulator of cell death mediated by tumour necrosis factor receptor 1 (TNFR1), as a target of EGLN1–pVHL. Prolyl hydroxylation of RIPK1 mediated by EGLN1 promotes the binding of RIPK1 with pVHL to suppress its activation under normoxic conditions. Prolonged hypoxia promotes the activation of RIPK1 kinase by modulating its proline hydroxylation, independent of the TNFα–TNFR1 pathway. As such, inhibiting proline hydroxylation of RIPK1 promotes RIPK1 activation to trigger cell death and inflammation. Hepatocyte-specific Vhl deficiency promoted RIPK1-dependent apoptosis to mediate liver pathology. Our findings illustrate a key role of the EGLN–pVHL pathway in suppressing RIPK1 activation under normoxic conditions to promote cell survival and a model by which hypoxia promotes RIPK1 activation through modulating its proline hydroxylation to mediate cell death and inflammation in human diseases, independent of TNFR1. Zhang, Xu, Liu, Wang et al. identify an inhibitory mechanism for RIPK1 kinase through EGLN1/pVHL-mediated proline hydroxylation, which is disrupted upon prolonged hypoxia that activates RIPK1 activity to promote cell death and inflammation.
长期缺氧减轻脯氨酰羟基化介导的RIPK1抑制,促进坏死和炎症。
由EGLN-pVHL途径介导的缺氧诱导因子1α(HIF-1α)的脯氨酰羟基化代表了一种经典的信号机制,其介导细胞在缺氧下的适应。在这里,我们确定RIPK1,一种由肿瘤坏死因子受体1(TNFR1)介导的细胞死亡的已知调节因子,作为EGLN1-pVHL的靶标。EGLN1介导的RIPK1的脯氨酸羟基化促进RIPK1与pVHL的结合,以在常氧条件下抑制其活化。长期缺氧通过调节其脯氨酸羟基化来促进RIPK1激酶的激活,不依赖于TNFα-TNFR1途径。因此,抑制RIPK1的脯氨酸羟基化促进RIPK1活化以触发细胞死亡和炎症。肝细胞特异性Vhl缺乏促进RIPK1依赖性细胞凋亡以介导肝脏病理。我们的研究结果阐明了EGLN-pVHL通路在常氧条件下抑制RIPK1激活以促进细胞存活的关键作用,以及缺氧通过调节其脯氨酸羟基化来促进RIPK1活化以介导人类疾病中的细胞死亡和炎症的模型,该模型独立于TNFR1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


