Redox Inversion: A Radical Analogue of Umpolung Reactivity for Base- and Metal-Free Catalytic C(sp3)–C(sp3) Coupling
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
The construction of alkyl–alkyl bonds is a powerful tool in organic synthesis. Redox inversion, defined as switching the donor/acceptor profile of a functional group to its acceptor/donor profile, is used for C(sp3)–C(sp3) coupling. We report a photocatalytic coupling of carboxylic acids to form bibenzyls through a radical–radical coupling. Mechanistic insight is gained through control reactions. This unexplored redox-opposite relationship between a carboxylic acid and its redox-active ester is implemented in catalysis.
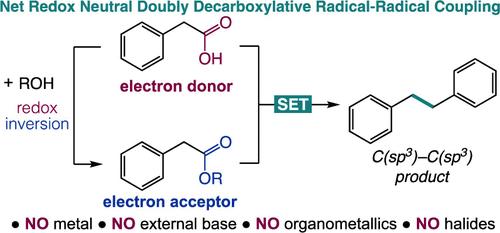
氧化还原转化:无碱和无金属催化C(sp3) - C(sp3)偶联Umpolung反应活性的自由基模拟
烷基-烷基键的构造是有机合成的有力工具。氧化还原反转,定义为将官能团的供体/受体结构转换为其受体/供体结构,用于C(sp3) -C (sp3)耦合。我们报道了羧酸通过自由基-自由基偶联形成联苯的光催化偶联。机械洞察力是通过控制反应获得的。羧酸和它的氧化还原活性酯之间的这种未探索的氧化还原-反关系在催化中实现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


