Gut microbiome immaturity and childhood acute lymphoblastic leukaemia
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Acute lymphoblastic leukaemia (ALL) is the most common cancer of childhood. Here, we map emerging evidence suggesting that children with ALL at the time of diagnosis may have a delayed maturation of the gut microbiome compared with healthy children. This finding may be associated with early-life epidemiological factors previously identified as risk indicators for childhood ALL, including caesarean section birth, diminished breast feeding and paucity of social contacts. The consistently observed deficiency in short-chain fatty-acid-producing bacterial taxa in children with ALL has the potential to promote dysregulated immune responses and to, ultimately, increase the risk of transformation of preleukaemic clones in response to common infectious triggers. These data endorse the concept that a microbiome deficit in early life may contribute to the development of the major subtypes of childhood ALL and encourage the notion of risk-reducing microbiome-targeted intervention in the future. In this Perspective article, Mel Greaves and co-workers outline emerging evidence that suggests that children with newly diagnosed acute lymphoblastic leukaemia may have a delayed maturation of the gut microbiome compared with healthy children, a deficit that might be associated with early-life epidemiological factors and could contribute to the risk of transformation of preleukaemic clones in response to common infectious triggers.
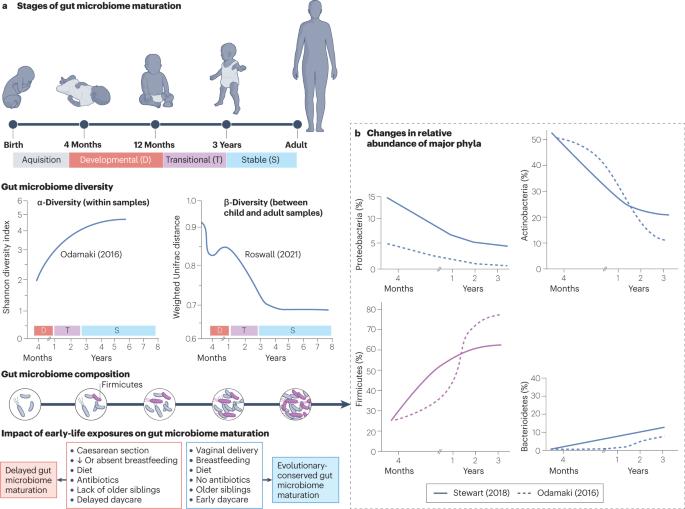
肠道微生物群不成熟与儿童急性淋巴细胞白血病
急性淋巴细胞白血病(ALL)是最常见的儿童癌症。在这里,我们绘制了新出现的证据,这些证据表明,与健康儿童相比,确诊时患有急性淋巴细胞白血病的儿童的肠道微生物组可能会延迟成熟。这一发现可能与之前被确定为儿童 ALL 风险指标的早期流行病学因素有关,这些因素包括剖腹产、母乳喂养减少和社会交往稀少。在儿童白血病患者中持续观察到的短链脂肪酸细菌类群的缺乏有可能导致免疫反应失调,并最终增加白血病前期克隆在常见感染诱因下发生转化的风险。这些数据证实了生命早期微生物组缺失可能导致儿童 ALL 主要亚型发展的观点,并鼓励了未来以降低风险的微生物组为目标进行干预的理念。在这篇 "视角 "文章中,梅尔-格里夫斯(Mel Greaves)及其合作者概述了新出现的证据,这些证据表明,与健康儿童相比,新确诊的急性淋巴细胞白血病患儿的肠道微生物组可能会延迟成熟,这种缺陷可能与生命早期的流行病学因素有关,并可能导致白血病前期克隆在常见感染诱因下发生转化的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


