Understanding tumour endothelial cell heterogeneity and function from single-cell omics
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 5
Abstract
Anti-angiogenic therapies (AATs) are used to treat different types of cancers. However, their success is limited owing to insufficient efficacy and resistance. Recently, single-cell omics studies of tumour endothelial cells (TECs) have provided new mechanistic insight. Here, we overview the heterogeneity of human TECs of all tumour types studied to date, at the single-cell level. Notably, most human tumour types contain varying numbers but only a small population of angiogenic TECs, the presumed targets of AATs, possibly contributing to the limited efficacy of and resistance to AATs. In general, TECs are heterogeneous within and across all tumour types, but comparing TEC phenotypes across tumours is currently challenging, owing to the lack of a uniform nomenclature for endothelial cells and consistent single-cell analysis protocols, urgently raising the need for a more consistent approach. Nonetheless, across most tumour types, universal TEC markers (ACKR1, PLVAP and IGFBP3) can be identified. Besides angiogenesis, biological processes such as immunomodulation and extracellular matrix organization are among the most commonly predicted enriched signatures of TECs across different tumour types. Although angiogenesis and extracellular matrix targets have been considered for AAT (without the hoped success), the immunomodulatory properties of TECs have not been fully considered as a novel anticancer therapeutic approach. Therefore, we also discuss progress, limitations, solutions and novel targets for AAT development. In this Review, Zeng et al. describe the recent single-cell omics studies that have revealed the heterogeneity of human tumour endothelial cells and demonstrate that the phenotypes of these cells extend beyond that of simply being angiogenic, an observation that could be translated into the clinic to improve upon the success rate of current anti-angiogenic therapies.
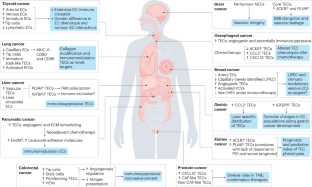
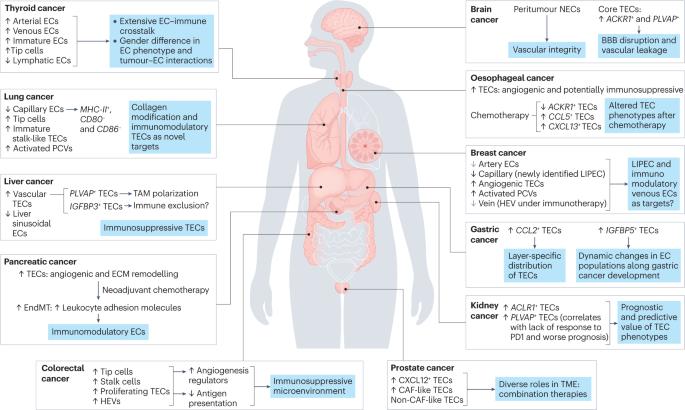
从单细胞全息技术了解肿瘤内皮细胞的异质性和功能
抗血管生成疗法(AATs)被用于治疗不同类型的癌症。然而,由于疗效不佳和耐药性,这些疗法的成功率有限。最近,对肿瘤内皮细胞(TECs)的单细胞全息研究提供了新的机理认识。在此,我们从单细胞水平概述了迄今为止研究的所有肿瘤类型的人类肿瘤内皮细胞的异质性。值得注意的是,大多数人类肿瘤类型都含有不同数量的血管生成TEC,但只有一小部分是AATs的假定靶点,这可能是导致AATs疗效有限和耐药性的原因。一般来说,所有肿瘤类型内部和之间的TEC都是异质性的,但由于缺乏统一的内皮细胞命名法和一致的单细胞分析方案,目前比较不同肿瘤的TEC表型具有挑战性,迫切需要一种更一致的方法。不过,在大多数肿瘤类型中,都能找到通用的 TEC 标记(ACKR1、PLVAP 和 IGFBP3)。除血管生成外,免疫调节和细胞外基质组织等生物过程也是不同肿瘤类型中最常见的TEC预测富集特征。虽然血管生成和细胞外基质靶点已被考虑用于 AAT(未取得预期成功),但 TECs 的免疫调节特性尚未被充分考虑作为一种新型抗癌治疗方法。因此,我们还讨论了 AAT 开发的进展、局限性、解决方案和新靶点。在这篇综述中,Zeng 等人介绍了最近的单细胞组学研究,这些研究揭示了人类肿瘤内皮细胞的异质性,并证明这些细胞的表型超出了单纯的血管生成表型,这一观察结果可应用于临床,以提高目前抗血管生成疗法的成功率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


