Four class A AUXIN RESPONSE FACTORs promote tomato fruit growth despite suppressing fruit set
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 3
Abstract
In flowering plants, auxin produced in seeds after fertilization promotes fruit initiation. The application of auxin to unpollinated ovaries can also induce parthenocarpy (seedless fruit production). Previous studies have shown that auxin signalling components SlIAA9 and SlARF7 (a class A AUXIN RESPONSE FACTOR (ARF)) are key repressors of fruit initiation in tomato (Solanum lycopersicum). A similar repressive role of class A ARFs in fruit set has also been observed in other plant species. However, evidence is lacking for a role of any class A ARF in promoting fruit development as predicted in the current auxin signalling model. Here we generated higher-order tomato mutants of four class A SlARFs (SlARF5, SlARF7, SlARF8A and SlARF8B) and uncovered their precise combinatorial roles that lead to suppressing and promoting fruit development. All four class A SlARFs together with SlIAA9 inhibited fruit initiation but promoted subsequent fruit growth. Transgenic tomato lines expressing truncated SlARF8A/8B lacking the IAA9-interacting PB1 domain displayed strong parthenocarpy, further confirming the promoting role of SlARF8A/8B in fruit growth. Altering the doses of these four SlARFs led to biphasic fruit growth responses, showing their versatile dual roles as both negative and positive regulators. RNA-seq and chromatin immunoprecipitation–quantitative PCR analyses further identified SlARF8A/8B target genes, including those encoding MADS-BOX transcription factors (AG1, MADS2 and AGL6) that are key repressors of fruit set. These results support the idea that SlIAA9/SlARFs directly regulate the transcription of these MADS-BOX genes to inhibit fruit set. Our study reveals the previously unknown dual function of four class A SlARFs in tomato fruit development and illuminates the complex combinatorial effects of multiple ARFs in controlling auxin-mediated fruit set and fruit growth. Mutant combinations of four AUXIN RESPONSE FACTORs in tomato by CRISPR–Cas9 technology reveal their dual function in inhibiting fruit set before pollination while activating fruit growth after fertilization.
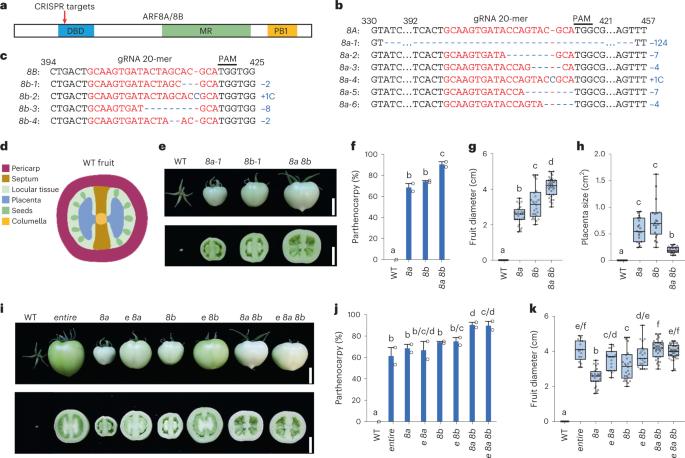
四种 A 类 AUXIN 反应因子尽管抑制坐果,但仍能促进番茄果实生长
在开花植物中,种子受精后产生的辅助素能促进果实的萌发。向未授粉的子房施用辅助素还能诱导孤雌生殖(无籽果实的产生)。先前的研究表明,在番茄(Solanum lycopersicum)中,辅助素信号成分 SlIAA9 和 SlARF7(A 类辅助素反应因子(ARF))是果实萌发的关键抑制因子。在其他植物物种中也观察到 A 类 ARF 在坐果过程中发挥类似的抑制作用。然而,目前还没有证据表明任何 A 类 ARF 在促进果实发育方面发挥了现有辅助素信号模型所预测的作用。在这里,我们产生了四个 A 类 SlARF(SlARF5、SlARF7、SlARF8A 和 SlARF8B)的高阶番茄突变体,并揭示了它们在抑制和促进果实发育方面的精确组合作用。所有四个A类SlARFs与SlIAA9一起抑制了果实的萌发,但促进了果实的后续生长。表达缺乏与IAA9相互作用的PB1结构域的截短SlARF8A/8B的转基因番茄品系表现出强烈的孤雌性,进一步证实了SlARF8A/8B在果实生长中的促进作用。改变这四种 SlARFs 的剂量会导致双相的果实生长反应,显示出它们作为负性和正性调节因子的多功能双重作用。RNA-seq和染色质免疫沉淀-定量PCR分析进一步确定了SlARF8A/8B的靶基因,包括那些编码MADS-BOX转录因子(AG1、MADS2和AGL6)的基因,它们是果实座果的关键抑制因子。这些结果支持了 SlIAA9/SlARFs 直接调控这些 MADS-BOX 基因转录以抑制坐果的观点。我们的研究揭示了四个 A 类 SlARFs 在番茄果实发育过程中之前未知的双重功能,并阐明了多个 ARFs 在控制辅助素介导的坐果和果实生长过程中的复杂组合效应。通过CRISPR-Cas9技术对番茄中的四种AUXIN RESPONSE FACTORs进行突变组合,揭示了它们在授粉前抑制坐果和受精后激活果实生长的双重功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


