The impact of adjunctive aripiprazole on QT interval: A 12-week open label study in patients on olanzapine, clozapine or risperidone
Abstract
Objective
To evaluate the effect of adjunct aripiprazole on QT of patients clinically stabilized on atypical antipsychotics.
Methods
The dataset was from an open-label 12-week prospective trial that evaluated adjunctive use of 5 mg/day of aripiprazole on metabolic profile in patients with schizophrenia, or schizoaffective disorder stabilized on olanzapine, clozapine, or risperidone. Bazett-corrected QT (QTc) was manually calculated from ECGs measured at baseline (before aripiprazole) and week 12, by two doctors blind to the diagnosis and atypical antipsychotic. The change in QTc (∆QTc: baseline QTc–week 12 QTc) and the number of participants in normal, borderline, prolonged, and pathological groups after 12 weeks were analyzed.
Results
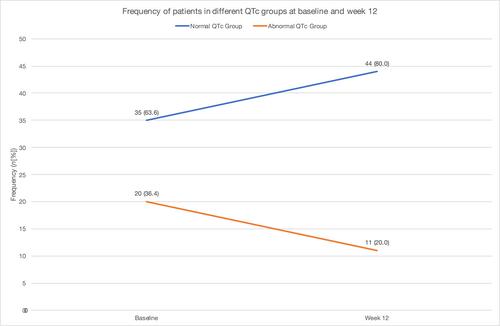
Fifty-five participants, mean age of 39.3 (SD 8.2) years, were analyzed. The ∆QTc after 12 weeks was 5.9 ms (p = 0.143) for the whole sample; 16.4 ms (p = 0.762), 3.7 ms (p = 0.480) and 0.5 ms (p = 0.449), for the clozapine, risperidone and olanzapine group, respectively. There was no significant statistical difference comparing the change in QTc overall, and between atypical antipsychotic groups, when evaluating from baseline to endpoint. However, stratifying the sample based on sex-dependent QTc cut-offs showed a 45% decrease in abnormal QTc readings (p = 0.049) after aripiprazole initiation; 20 subjects had abnormal QTc at baseline, while only 11 subjects had abnormal QTc at 12 weeks. 25.5% of participants showed a reduction in at least one QTc severity group, while 65.5% had no change and 9.0% worsened in QTc group, after 12 weeks of adjunct aripiprazole.
Conclusion
Low-dose adjunctive aripiprazole did not prolong QTc in patients stabilized on either olanzapine, risperidone, or clozapine. More controlled studies evaluating the QTc effect of adjunctive aripiprazole should be done to confirm and support these findings.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


